Abstract
Purpose
Intensive screening in BRCA1/2 mutation carriers aims to improve breast cancer (BC) prognosis. Our aim is to clarify the prognostic impact of tumor size in BRCA mutation carriers with a pT1 BC, which is currently unclear. We are especially interested in differences between pT1a, pT1b, and pT1c regarding the prognosis of node-negative breast cancer, the effect of chemotherapy, and the prevalence of lymph node involvement.
Methods
For this study, BRCA1/2-associated BC patients were selected from a nationwide cohort. Primary outcomes were 10-year overall survival (OS) per pT1a-b-c group and the effect of chemotherapy on prognosis of node-negative BC, using Kaplan–Meier and Cox models. Finally, we evaluated lymph node involvement per pT1a-b-c group.
Results
963 women with pT1 BRCA1/2-associated BC diagnosed between 1990 and 2017 were included, of which 679 had pN0 BC. After a median follow-up of 10.5 years, 10-year OS in patients without chemotherapy was 77.1% in pT1cN0 and lower than for pT1aN0 (91.4%, p = 0.119) and pT1bN0 (90.8%, p = 0.024). OS was better with than without chemotherapy for pT1cN0 (91.6% vs. 77.1%, p = 0.001; hazard ratio (HR) 0.56, 95% confidence interval (CI): 0.21–1.48). Lymph node involvement was 24.9% in pT1c, 18.8% in pT1b, and 8.6% in pT1a.
Conclusion
Smaller tumor size is associated with better OS and less lymph node involvement in pT1 BRCA1/2-associated BC patients. The results suggest that early detection in BRCA1/2 mutation carriers of pT1a/b BC may reduce mortality and the need for systemic therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Women carrying a pathogenic germline BRCA1 or BRCA2 mutation have life-time breast cancer (BC) risks up to 75%, and are often diagnosed with BC at a relatively young age [1]. Therefore, BRCA1/2 mutation carriers may opt for bilateral prophylactic mastectomy, reducing BC risk to almost zero, or participate in a tailored BC screening program [2]. The purpose of screening is to find BC in an early stage with excellent prognosis, preferably without the necessity of endocrine therapy or (neo)adjuvant chemotherapy. A prerequisite for the latter is the absence of nodal involvement.
In the general population, randomized controlled trials (RCT) of mammography screening demonstrated a reduction in BC mortality [3]. Consequently, the general consensus is that actively trying to find BC at an early stage is effective, and a nationwide screening program for women aged 50–75 years was implemented in many European countries. The basis for effective screening lies in the fact that sporadic BC patients with a small node-negative tumor have an excellent prognosis [4]. Tumor size and lymph node involvement are positively correlated and both are independent predictors for BC-related mortality [5, 6]. These findings reinforce the rationale behind population-wide screening programs. For BRCA1/2 mutation carriers, however, no trials investigating screening exist, and screening efficacy is only presumed to be similar to that of the general population [7]. At the age of 25—the recommended age to start BC screening for BRCA1/2 mutation carriers—breast density is higher than for women in regular screening programs, possibly affecting mammography efficacy. Therefore, and to avoid radiation exposure, MRI is already the modality of choice in young women. Additionally, debate is still ongoing whether the association between tumor size and outcome is as strongly present in BRCA1/2-associated BC [8,9,10,11]. Moreover, the correlation between tumor size and lymph node involvement in BRCA1 mutation carriers has been reported to be weaker than for sporadic BC or BRCA2-associated BC [12]. Together, these findings imply uncertainty regarding survival benefit from BC screening in BRCA1/2 mutation carriers.
Screening programs tailored to BRCA1/2 mutation carriers have previously been investigated, but so far no studies could directly demonstrate survival benefit from BC screening [13, 14]. Given that data on the prognostic value of tumor size in BRCA1/2 mutation carriers are currently lacking, the purpose of the current study is to determine the prognostic value of this tumor characteristic within a population of BRCA1/2 mutation carriers. First, we evaluate whether tumor size, categorized as pT1a (0.1–0.5 cm), pT1b (> 0.5–1.0 cm), and pT1c (> 1.0–2.0 cm) according to the TNM classification of malignant tumors, UICC (TNM), is a good prognostic factor of survival in pT1N0 BRCA1/2-associated BC patients who did not receive chemotherapy. We are especially interested in the natural course of disease without chemotherapy and the possibilities to omit this treatment modality for patients with small node-negative tumors, for chemotherapy can severely impact quality of life. Second, we evaluate the effect of chemotherapy in BRCA1/2-associated BC. Third, as lymph node involvement has consequences for both treatment (regardless of tumor size), and prognosis in the general BC population, we evaluate the association between tumor size and lymph node status in BRCA1/2-associated BC patients.
Patients and methods
Eligible participants were retrieved from the ongoing national HEBON (Hereditary Breast and Ovarian Cancer Research Netherlands) study cohort. In the HEBON study, members of families with pathogenic germline BRCA1/2 mutations were identified through the departments of Clinical Genetics of the eight Dutch academic medical centers and the Netherlands Cancer Institute. All participating centers’ Medical Ethics Committees approved the study. Written informed consent was acquired from each participating woman, or a close relative or proxy for deceased individuals. Relevant data on patient characteristics, cancer diagnosis, tumor characteristics, and preventive strategies were retrieved and updated through linkage with the Dutch National Cancer Registry and the national pathology database, and from medical files and questionnaires. Therefore, this registry is a mixture of both retrospective data collection and prospective follow-up [15]. The latest follow-up date used in the current study was December 31, 2017.
We included only women carrying a proven pathogenic germline BRCA1 or BRCA2 mutation via testing, and diagnosed with pT1a, pT1b, or pT1c breast cancer between 1 January 1990 and 1 January 2017 (regardless of N-status). BC was diagnosed either before or after confirmation of BRCA1/2 mutation carrier status. Patients were excluded if they had distant metastases at BC diagnosis, had received neoadjuvant chemotherapy (i.e., lacking pT assessment), or had any other primary tumor prior to BC diagnosis (except cervical intraepithelial neoplasia (CIN), skin basal cell carcinoma (BCC) or skin squamous cell carcinoma (SCC)). Further reasons for exclusion were insufficient data regarding tumor size, vital status, or date of death.
Data collection
We retrieved dates of birth, BC diagnosis, DNA test, and death. We retrieved information on TNM classification current at the time of diagnosis, mode of detection, estrogen receptor (ER) status, progesterone receptor (PR) status, Human Epidermal Growth Factor Receptor 2 (HER2)-status, type of surgery, chemotherapy, HER2-targeted treatment, endocrine treatment, radiotherapy, risk-reducing salpingo-oophorectomy (RRSO), risk-reducing mastectomy (RRM), BRCA mutation status, and vital status. All reported tumor stadia were histologically determined. ER-status and PR-status were defined positive if immunohistochemical staining showed 10% or more of the tumor cells positive for the receptor. The HER2-status was positive if the immunohistochemical staining was 3 + , or 2 + with a positive in-situ hybridization (ISH) test.
Statistical analyses
The primary outcome was 10-year overall survival (OS) from date of BC diagnosis. To allow for prevalent cases at date of genetic testing, we applied left truncation in the survival analyses [16]. Time at risk therefore started at date of BC diagnosis or of DNA test result, whichever came last, and ended at date of death or date of censoring event. This does not change the moment 10-year OS is evaluated, but modifies the time at risk during this 10-year period. Censoring events were any non-breast primary malignancy (with the exception of CIN, BCC and SCC) or the last date of follow-up.
We used Kaplan–Meier survival analysis and the Log-Rank test to compare unadjusted OS curves of the pT1aN0, pT1bN0, and pT1cN0 subgroups not receiving chemotherapy, and of patients receiving chemotherapy vs no chemotherapy within the pT1N0 subgroups. Cox proportional hazards regression models were used to calculate hazard ratios (HR) and 95% confidence intervals (95% CI) for OS. We applied a subject matter knowledge-based model-building strategy, as we were interested in the etiological impact of tumor size on prognosis, and not in prediction. The following relevant clinical or pathological variables were included in the multivariable model: mutational status, ER-status, HER2-status, chemotherapy, endocrine therapy, RRSO, year of diagnosis, and age at diagnosis.
Finally, we also investigated the percentage of lymph node involvement at time of BC diagnosis, stratified by tumor size. As early detection is a major determinant of lymph node involvement, we further stratified by screening status. Missing data for screening were imputed by using the timing of DNA test result respective of BC diagnosis, as women with a DNA test result before BC diagnosis are very likely to participate in screening [17].
Descriptive statistics are shown as proportions or median and range. We used Pearson’s χ2 test to compare categorical variables and the Kruskal–Wallis test for continuous variables. All p values are two-sided.
Statistical analyses were performed using STATA 15.1.
Because of missing data for several important covariables (such as the HER2-status), we implemented a multiple imputation model to allow for all variables to be included in the multivariable Cox models without losing observations. The imputation model was built in STATA 15.1, see supplemental materials (Online Resource 1).
The models were checked for interactions between the main variable of interest with all other included variables. We tested the proportional hazards assumption through the addition of time-varying effects to the model, using a cut-off of p < 0.05 for the time-varying term.
Results
Patient characteristics
We included 963 women with primary BRCA1/2-associated pT1 BC (Fig. 1). A total of 679 women had lymph node-negative disease at diagnosis (70.5%), of whom 51 (7.5%) were diagnosed with a pT1a tumor, 159 (23.4%) with a pT1b tumor, and 469 (69.1%) with a pT1c tumor. A full overview of patient, tumor, and treatment characteristics of the node-negative patients is provided in Table 1. Median follow-up time of the node-negative cohort was 10.5 years, and median age at BC diagnosis was 42.6 years. Patients with a pT1cN0 tumor received chemotherapy in 60.1% of cases, compared to 34.0% of pT1bN0 patients and 15.7% of pT1aN0 patients. BRCA1 mutation carriers more often had a larger tumor than BRCA2 mutation carriers (pT1c 71.6% vs 63.3%, p = 0.030, data not shown) and more often had an ER-negative tumor (79.7% vs 26.4%, p < 0.001, data not shown).
Ten-year overall survival in pT1N0 breast cancer
In women who did not receive chemotherapy, 10-year OS was 77.1% for pT1c patients and lower than for pT1a (91.4%; p = 0.08) and pT1b (90.8%; p = 0.02) patients (Table 2; Fig. 2). When stratified by BRCA1/2 mutation, a similar OS was seen for the different tumor sizes, except for pT1b (97.4% 10-year OS in BRCA1 vs 82.8% in BRCA2, p = 0.049)(Table 2). Among pT1N0 chemotherapy recipients (BRCA1 and BRCA2 combined), 10-year OS of pT1a patients (69.4%) was worse than for pT1b (100%, p < 0.001) and pT1c patients (91.6%, p = 0.04)(Table 2).
For pT1cN0 and pT1bN0 patients, OS was better with chemotherapy than without (91.6% vs. 77.1%, p = 0.001, and 100% vs 90.8%, p = 0.11, respectively). In pT1aN0 patients, OS was worse for those receiving chemotherapy than for those without (69.4% vs 91.4%, p = 0.008)(Table 2).
Adjusted survival analyses
For node-negative patients not receiving chemotherapy, adjusted HRs for overall mortality of 0.71 (95% CI 0.14–3.51) for pT1a and of 0.36 (95% CI 0.14–0.94) for pT1b were found when compared to pT1c patients (Table 3). Among pT1cN0 patients, chemotherapy compared to none revealed an adjusted HR for overall mortality of 0.56 (95% CI 0.21–1.48)(Table 3).
Lymph node involvement
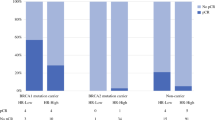
In the total pT1 population (n = 963), 28.5% of patients had lymph node involvement at diagnosis. The proportion with positive lymph nodes increased with larger tumor size at diagnosis: 16.4% for pT1a, 20.9% for pT1b, and 32.1% for pT1c (data not shown). In the screened population (n = 322, 33.4%), we found 20.9% lymph node involvement (8.6% for pT1a, 18.8% for pT1b, and 24.9% for pT1c; Fig. 3). When evaluated separately, in both BRCA1 and BRCA2 mutation carriers, pT1b and pT1c patients more often had lymph node involvement at diagnosis than pT1a patients (Online Resource 1). The highest proportion of lymph node involvement was found in BRCA2 mutation carriers with a pT1c tumor (Online Resource 1).
Percentage lymph node involvement in screened and non-screened patients. *known to be screened or not, missing values imputed based on DNA diagnosis (see methods for details). Abbreviations: pN, pathological lymph node assessment (pN ± lymph nodes are tumor-positive); pT, pathological tumor assessment (pT1a = 0.1–0.5 cm, pT1b ≥ 0.5–1.0 cm, pT1c ≥ 1.0–2.0 cm)
Discussion
The results of our study showed that, as is the case for non-hereditary BC, smaller tumor size was associated with improved OS in pT1N0M0 BRCA1/2-associated BC patients. We found a strong indication that adjuvant chemotherapy improves survival for patients with a pT1cN0 tumor. Twenty-nine percent of the total population had lymph node involvement, its prevalence increasing with larger tumor size at diagnosis.
Our results in pT1N0 BC patients contradict those of previous studies. Narod et al. observed no significant difference in OS between BRCA1 mutation carriers with node-negative tumors of 0.1–1 cm and of 1–2 cm (adjusted HR 1.39, 95% CI 0.67–2.90) [8]. Similarly, Huzarski et al. found no significant association for BRCA1 mutation carriers between tumor size and OS within the pT1 subgroup (adjusted HR 0.92, 95% CI 0.33–2.57, for tumors > 1 cm) [9]. This study included only 40 patients with a pT1a or pT1b tumor, limiting the probability of finding a difference. Another explanation for the different results, however, is that Huzarski et al. included patients treated with neo-adjuvant chemotherapy (66 out of 233), thereby potentially misclassifying clinically larger tumors as pT1, resulting in HRs closer to 1.00.
Systemic therapy can improve outcome of pT1 sporadic BC, although only of selected subgroups, and the effect might be small [18, 19]. A study among pT1 node-negative BRCA1-associated BC patients suggested that patients who did not receive chemotherapy appeared to have worse overall survival (OS) than patients who did receive chemotherapy [8]. While the effect is both small and limited to specific subgroups in sporadic patients, BRCA1 mutation carriers with pT1N0 BC appear to benefit more from chemotherapy. One of the explanations for this might be that BC in BRCA1 mutation carriers more often is of the triple-negative or basal-like phenotype, and of higher histologic grade [20, 21]. Therefore, more patients with pT1 BRCA1-associated BC could benefit from chemotherapy than previously assumed, even when detected by screening in an early stage. While we cannot demonstrate a direct benefit of chemotherapy, we did see that those treated with chemotherapy had better OS than those not treated with chemotherapy, with the exception of women with a pT1a tumor. Considering the small proportion of pT1aN0 patients receiving chemotherapy, this finding may simply be due to chance. Caution is warranted however, as we do not know the reason why physicians chose to advise adjuvant chemotherapy for some, but not all patients. Consequently, the chemotherapy and non-chemotherapy groups may not be comparable in terms of prognosis.
Despite screening, still 8.6% of pT1a, 18.8% of pT1b, and 24.9% of pT1c BC patients had positive nodes at diagnosis. This appears to be similar to the general population [22,23,24], but is higher than recently reported based on SEER data from 2010 to 2014. In that study, the overall lymph node positivity ranged from 1.4% for pT1a to 6.0% for pT1c [25]. We can only speculate on explanations for this remarkable difference. Possibly, as the latter study describes more recent data than ours, a stage-shift may have occurred with improving imaging techniques over the years, resulting in earlier BC detection with more smaller and node-negative tumors. Another explanation may be that BRCA-associated breast tumors show a different biological background than tumors among the general population (as used in the study by Zhao et al. [25]). Indeed, we observed especially among BRCA2 mutation carriers high rates of lymph node involvement. The positive association between lymph node involvement and tumor size appears to be stronger in BRCA2 mutation carriers than in BRCA1 mutation carriers. Earlier work by Foulkes et al. also showed a significant positive correlation between tumor size and lymph node involvement for BRCA2-associated BC. However, they observed no clear association among BRCA1-associated BC patients [12]. This may have been due to smaller sample sizes. The observation that lymph node involvement is more frequent in BRCA2 mutation carriers may be the result of tumor biology. Numerous reports suggest that hormone receptor-positive BC is indeed more likely to spread to the lymph nodes than triple-negative BC [26,27,28,29]. In our population, 78.8% of the BRCA2 mutation carriers were diagnosed with a hormone receptor-positive BC, compared to only 24.2% of the BRCA1 mutation carriers (p < 0.001).
One of the strengths of our study was the ability to assemble a large cohort of BRCA1/2-associated BC patients, with a relatively long follow-up. Furthermore, studies directly investigating the impact of screening usually have to deal with length–time bias and lead-time bias [30]. Because we primarily investigated the effect of tumor size (and not screening) on survival, these biases are unlikely to have affected our results. Lead-time bias could in theory still apply if there is a large screening differential among the tumor size groups. However, 10-year OS of pT1cN0 was worse compared to pT1bN0 and comparable to pT1aN0, irrespective of screening (89.5% compared to 96.8% and 90.7% with screening, 85.4% compared to 90.1% and 84.6% without screening, data not shown). Although we do see a (non-significant) change in OS with increasing tumor size, the absolute OS differences between pT1 subgroups remain fairly constant in both screened and unscreened patients, and therefore do not indicate the presence of (meaningful) bias. It should be noted that screening efficacy could be influenced by the fact that RRSO may decrease breast density. In the current cohort, uptake of RRSO before BC diagnosis was higher in pT1a/pT1b patients than in pT1c patients. Denser breast tissue may have delayed diagnosis of BC in the pT1c group. Possibly, if pT1c patients had undergone RRSO before a BC diagnosis, they might have been diagnosed with a pT1a or pT1b tumor instead.
A minor limitation is that we did not have a cohort of sporadic BC cases to directly compare our results with. Previously, several studies compared OS or breast cancer-specific survival (BCSS) between BRCA1/2-associated and sporadic BC. It is currently unclear whether overall survival is worse for BRCA mutation carriers, but if assumed to be the case, this may be largely due to the higher incidence of triple-negative BC (TNBC) within the BRCA1 population as well as the potential for survival bias [31,32,33,34]. Further, a high incidence of TNBC in especially BRCA1 mutation carriers may result in another complication. We used left-truncation to minimize survival bias from BC patients who were tested for BRCA mutation after their BC diagnosis. However, because the hazard for death is especially high in the first few years after diagnosis of TNBC, selection of favorable TNBC cases may still occur when the interval between BC diagnosis and testing is long. While this did not seem to be the case for our cohort (median time to testing 1.6 years, data not shown), one can never truly know which patients are missing due to selection bias, and the survival rates reported here may be an overestimation. However, comparing survival of only those who were tested for a BRCA mutation before breast cancer diagnosis (prospective analysis), we found that the 10-year survival rates are 100% for pT1aN0, 98.3% for pT1b, and 89.2% for pT1c. All are higher than what we found for the whole cohort (Table 2: 87.5%, 93.4% and 86.2% for pT1aN0, pT1bN0, and pT1cN0 respectively). In our opinion, this makes it less likely that survival is overestimated for newly diagnosed carriers.
This also suggests that BRCA1 and BRCA2 mutation carriers should ideally be analyzed separately, due to their tumor’s biological differences. However, despite having a much larger population of BRCA mutation carriers than any study before, this would still result in subgroups too small to draw reasonable conclusions. Instead, we opted to adjust the multivariable models for these biological differences, as well mutation status (i.e., BRCA1 or BRCA2). A more important limitation was the small number of pT1a patients, making it difficult to draw useful conclusions about their prognosis. Combining the pT1a and pT1b groups into a 0.1–1.0 cm category could improve power, but would only allow for a generalized clinical application. When combined, we find 10-year survival rates of 91.9% for pT ≤ 1 cm (T1aN0/T1bN0) and 86.2% for pT > 1 cm (p = 0.02). For chemotherapy recipients, 10-year survival rates were 96.0% for pT ≤ 1 cm and 91.6% for pT > 1 cm. Among patients not receiving chemotherapy, 10-year survival was 91.0% for pT ≤ 1 cm and 77.1% for pT > 1 cm (p = 0.006), adjusted hazard ratio 0.40 (0.16–0.99).
Another limitation arose as a result from the median year of BC diagnosis in our cohort being in the early 2000s, with hormone receptor status and especially HER2-status missing for a substantial proportion of cases. Therefore, we could not use TNBC as a variable for stratification. Instead, we used the imputed variables ER-status and HER2-status for adjustment. Further, the cause of death was unknown for a large proportion of cases, making BCSS analyses impossible. However, because we had a relatively young cohort with a median age of 42.3 years at BC diagnosis, and we censored at diagnosis of another cancer (including ovarian cancer), we can assume that the majority of the unknown causes of death are BC related. Finally, although a complete screening variable would be preferred, we expect our practical solution of a DNA-test result-based imputation provides a close enough approximation for our analysis on lymph node involvement where screening was taken into account.
Ultimately, we observed (1) better prognosis with a smaller tumor size at diagnosis, (2) possibly improved survival after adjuvant chemotherapy treatment for those with a pT1bN0 or pT1cN0 tumor, and (3) less lymph node involvement at diagnosis for those with a smaller tumor size. These findings confirm several potential benefits from intensive screening for women at high risk of developing BC due to a BRCA1/2 mutation, under the assumption that screening indeed leads to finding BC when the tumor is small and before lymph node involvement occurs.
In conclusion, overall survival of BRCA1/2-associated breast cancer patients is better when they are diagnosed with a smaller tumor size within the pT1 category. Lymph node involvement is a frequent occurrence in BRCA1/2-associated BC and increases with larger tumor size. The results support current intensive screening strategies in BRCA1/2 mutation carriers, aiming to detect preferentially pT1a/b BC to improve survival and reduce the need for systemic therapy. To achieve early detection more often, research into further optimization of imaging techniques may be warranted.
Data availability
The data underlying this article are not publicly available due to the presence of highly sensitive patient information, but may be shared on reasonable request to the Hebon Steering Committee, when it does not conflict with Hebon policy or Dutch privacy law (GDPR).
Abbreviations
- BCC:
-
Basal cell carcinoma of the skin
- BC:
-
Breast cancer
- BCSS:
-
Breast cancer-specific survival
- CIN:
-
Cervical intraepithelial neoplasia
- CI:
-
Confidence interval
- ER:
-
Estrogen receptor
- HR:
-
Hazard ratio
- Hebon:
-
Hereditary Breast and Ovarian Cancer Research Netherlands
- HER2:
-
Human Epidermal Growth Factor Receptor 2
- ISH:
-
In-situ hybridization
- MRI:
-
Magnetic Resonance Imaging
- OS:
-
Overall survival
- PR:
-
Progesterone receptor
- RCT:
-
Randomized controlled trial
- RRM:
-
Risk-reducing mastectomy
- RRSO:
-
Risk-reducing salpingo-oophorectomy
- SCC:
-
Squamous cell carcinoma of the skin
- TNM:
-
TNM (Tumor Nodes Metastasis) classification of malignant tumors, UICC
- TNBC:
-
Triple-negative breast cancer
References
Mavaddat N, Peock S, Frost D, Ellis S, Platte R, Fineberg E et al (2013) Cancer risks for BRCA1 and BRCA2 mutation carriers: results from prospective analysis of EMBRACE. J Natl Cancer Inst 105(11):812–822. https://doi.org/10.1093/jnci/djt095
Tung N (2011) Management of women with BRCA mutations: a 41-year-old woman with a BRCA mutation and a recent history of breast cancer. JAMA 305(21):2211–2220. https://doi.org/10.1001/jama.2011.678
Kerlikowske K, Grady D, Rubin SM, Sandrock C, Ernster VL (1995) Efficacy of screening mammography A meta-analysis. JAMA 273(2):149–154. https://doi.org/10.1001/jama.1995.03520260071035
Parise CA, Caggiano V (2017) Risk of mortality of node-negative, ER/PR/HER2 breast cancer subtypes in T1, T2, and T3 tumors. Breast Cancer Res Treat 165(3):743–750. https://doi.org/10.1007/s10549-017-4383-5
Saadatmand S, Bretveld R, Siesling S, Tilanus-Linthorst MM (2015) Influence of tumour stage at breast cancer detection on survival in modern times: population based study in 173,797 patients. BMJ 351:h4901. https://doi.org/10.1136/bmj.h4901
Narod SA (2012) Tumour size predicts long-term survival among women with lymph node-positive breast cancer. Curr Oncol 19(5):249–253. https://doi.org/10.3747/co.19.1043
Saadatmand S, Obdeijn IM, Rutgers EJ, Oosterwijk JC, Tollenaar RA, Woldringh GH et al (2015) Survival benefit in women with BRCA1 mutation or familial risk in the MRI screening study (MRISC). Int J Cancer 137(7):1729–1738. https://doi.org/10.1002/ijc.29534
Narod SA, Metcalfe K, Lynch HT, Ghadirian P, Robidoux A, Tung N et al (2013) Should all BRCA1 mutation carriers with stage I breast cancer receive chemotherapy? Breast Cancer Res Treat 138(1):273–279. https://doi.org/10.1007/s10549-013-2429-x
Huzarski T, Byrski T, Gronwald J, Gorski B, Domagala P, Cybulski C et al (2013) Ten-year survival in patients with BRCA1-negative and BRCA1-positive breast cancer. J Clin Oncol 31(26):3191–3196. https://doi.org/10.1200/JCO.2012.45.3571
Rennert G, Bisland-Naggan S, Barnett-Griness O, Bar-Joseph N, Zhang S, Rennert HS et al (2007) Clinical outcomes of breast cancer in carriers of BRCA1 and BRCA2 mutations. N Engl J Med 357(2):115–123. https://doi.org/10.1056/NEJMoa070608
Goodwin PJ, Phillips KA, West DW, Ennis M, Hopper JL, John EM et al (2012) Breast cancer prognosis in BRCA1 and BRCA2 mutation carriers: an International Prospective Breast Cancer Family Registry population-based cohort study. J Clin Oncol 30(1):19–26. https://doi.org/10.1200/JCO.2010.33.0068
Foulkes WD, Metcalfe K, Hanna W, Lynch HT, Ghadirian P, Tung N et al (2003) Disruption of the expected positive correlation between breast tumor size and lymph node status in BRCA1-related breast carcinoma. Cancer 98(8):1569–1577. https://doi.org/10.1002/cncr.11688
Warner E, Zhu S, Plewes DB, Hill K, Ramsay EA, Causer PA et al (2020) Breast cancer mortality among women with a BRCA1 or BRCA2 mutation in a magnetic resonance imaging plus mammography screening program. Cancers (Basel). 12(11):10. https://doi.org/10.3390/cancers12113479
Moller P, Stormorken A, Jonsrud C, Holmen MM, Hagen AI, Clark N et al (2013) Survival of patients with BRCA1-associated breast cancer diagnosed in an MRI-based surveillance program. Breast Cancer Res Treat 139(1):155–161. https://doi.org/10.1007/s10549-013-2540-z
Pijpe A, Manders P, Brohet RM, Collee JM, Verhoef S, Vasen HF et al (2010) Physical activity and the risk of breast cancer in BRCA1/2 mutation carriers. Breast Cancer Res Treat 120(1):235–244. https://doi.org/10.1007/s10549-009-0476-0
Azzato EM, Greenberg D, Shah M, Blows F, Driver KE, Caporaso NE et al (2009) Prevalent cases in observational studies of cancer survival: do they bias hazard ratio estimates? Br J Cancer 100(11):1806–1811. https://doi.org/10.1038/sj.bjc.6605062
Metcalfe K, Eisen A, Senter L, Armel S, Bordeleau L, Meschino WS et al (2019) International trends in the uptake of cancer risk reduction strategies in women with a BRCA1 or BRCA2 mutation. Br J Cancer 121(1):15–21. https://doi.org/10.1038/s41416-019-0446-1
Ignatov T, Eggemann H, Burger E, Costa SD, Ignatov A (2017) Management of small T1a/b breast cancer by tumor subtype. Breast Cancer Res Treat 163(1):111–118. https://doi.org/10.1007/s10549-017-4168-x
Vaz-Luis I, Ottesen RA, Hughes ME, Mamet R, Burstein HJ, Edge SB et al (2014) Outcomes by tumor subtype and treatment pattern in women with small, node-negative breast cancer: a multi-institutional study. J Clin Oncol 32(20):2142–2150. https://doi.org/10.1200/JCO.2013.53.1608
Honrado E, Benitez J, Palacios J (2005) The molecular pathology of hereditary breast cancer: genetic testing and therapeutic implications. Mod Pathol 18(10):1305–1320. https://doi.org/10.1038/modpathol.3800453
Brekelmans CT, Tilanus-Linthorst MM, Seynaeve C, vd Ouweland A, Menke-Pluymers MB, Bartels CC, et al. Tumour characteristics, survival and prognostic factors of hereditary breast cancer from BRCA2-, BRCA1- and non-BRCA1/2 families as compared to sporadic breast cancer cases. Eur J Cancer. 2007;43(5):867–76. https://doi.org/10.1016/j.ejca.2006.12.009
Laura S, Coombs NJ, Ung O, Boyages J (2006) Tumour size as a predictor of axillary node metastases in patients with breast cancer. ANZ J Surg 76(11):1002–1006. https://doi.org/10.1111/j.1445-2197.2006.03918.x
Sopik V, Narod SA (2018) The relationship between tumour size, nodal status and distant metastases: on the origins of breast cancer. Breast Cancer Res Treat 170(3):647–656. https://doi.org/10.1007/s10549-018-4796-9
Chua B, Ung O, Taylor R, Boyages J (2001) Frequency and predictors of axillary lymph node metastases in invasive breast cancer. ANZ J Surg 71(12):723–728. https://doi.org/10.1046/j.1445-1433.2001.02266.x
Zhao YX, Liu YR, Xie S, Jiang YZ, Shao ZM (2019) A nomogram predicting lymph node metastasis in T1 breast cancer based on the surveillance, epidemiology, and end results program. J Cancer 10(11):2443–2449. https://doi.org/10.7150/jca.30386
He ZY, Wu SG, Yang Q, Sun JY, Li FY, Lin Q et al (2015) Breast cancer subtype is associated with axillary lymph node metastasis: a retrospective cohort study. Medicine (Baltimore) 94(48):e2213. https://doi.org/10.1097/MD.0000000000002213
Mattes MD, Bhatia JK, Metzger D, Ashamalla H, Katsoulakis E (2015) Breast Cancer Subtype as a Predictor of Lymph Node Metastasis according to the SEER Registry. J Breast Cancer 18(2):143–148. https://doi.org/10.4048/jbc.2015.18.2.143
Crabb SJ, Cheang MC, Leung S, Immonen T, Nielsen TO, Huntsman DD et al (2008) Basal breast cancer molecular subtype predicts for lower incidence of axillary lymph node metastases in primary breast cancer. Clin Breast Cancer 8(3):249–256. https://doi.org/10.3816/CBC.2008.n.028
Reyal F, Rouzier R, Depont-Hazelzet B, Bollet MA, Pierga JY, Alran S et al (2011) The molecular subtype classification is a determinant of sentinel node positivity in early breast carcinoma. PloS ONE 6(5):e20297. https://doi.org/10.1371/journal.pone.0020297
Duffy SW, Nagtegaal ID, Wallis M, Cafferty FH, Houssami N, Warwick J et al (2008) Correcting for lead time and length bias in estimating the effect of screen detection on cancer survival. Am J Epidemiol 168(1):98–104. https://doi.org/10.1093/aje/kwn120
Baretta Z, Mocellin S, Goldin E, Olopade OI, Huo D (2016) Effect of BRCA germline mutations on breast cancer prognosis: a systematic review and meta-analysis. Medicine (Baltimore) 95(40):e4975. https://doi.org/10.1097/MD.0000000000004975
Copson ER, Maishman TC, Tapper WJ, Cutress RI, Greville-Heygate S, Altman DG et al (2018) Germline BRCA mutation and outcome in young-onset breast cancer (POSH): a prospective cohort study. Lancet Oncol 19(2):169–180. https://doi.org/10.1016/S1470-2045(17)30891-4
Tung N, Gaughan E, Hacker MR, Lee LJ, Alexander B, Poles E et al (2014) Outcome of triple negative breast cancer: comparison of sporadic and BRCA1-associated cancers. Breast Cancer Res Treat 146(1):175–182. https://doi.org/10.1007/s10549-014-2995-6
Schmidt MK, van den Broek AJ, Tollenaar RA, Smit VT, Westenend PJ, Brinkhuis M et al (2017) Breast cancer survival of BRCA1/BRCA2 mutation carriers in a hospital-based cohort of young women. J Natl Cancer Inst 109(8):10. https://doi.org/10.1093/jnci/djw329
Acknowledgements
The Hereditary Breast and Ovarian Cancer Research Group Netherlands (HEBON) consists of the following Collaborating Centers: Netherlands Cancer Institute (coordinating center), Amsterdam, NL: M.A. Rookus, F.B.L. Hogervorst, F.E. van Leeuwen, M.A. Adank, M.K. Schmidt, D.J. Stommel-Jenner, R. de Groot; Erasmus Medical Center, Rotterdam, NL: J.M. Collée, A.M.W. van den Ouweland, M.J. Hooning, I.A. Boere; Leiden University Medical Center, NL: C.J. van Asperen, P. Devilee, R.B. van der Luijt, T.C.T.E.F. van Cronenburg; Radboud University Nijmegen Medical Center, NL: M.R. Wevers, A.R. Mensenkamp; University Medical Center Utrecht, NL: M.G.E.M. Ausems, M.J. Koudijs; Amsterdam UMC, Univ of Amsterdam, NL: I. van de Beek; Amsterdam UMC, Vrije Universiteit Amsterdam, NL: K. van Engelen, J.J.P. Gille; Maastricht University Medical Center, NL: E.B. Gómez García, M.J. Blok, M. de Boer; University of Groningen, NL: L.P.V. Berger, A.H. van der Hout, M.J.E. Mourits, G.H. de Bock; The Netherlands Comprehensive Cancer Organization (IKNL): S. Siesling, J. Verloop; The nationwide network and registry of histo- and cytopathology in The Netherlands (PALGA): E.C. van den Broek. The HEBON study is supported by the Dutch Cancer Society [Grant Nos. NKI1998-1854, NKI2004-3088, NKI2007-3756, NKI2019-12535], the Netherlands Organization of Scientific Research [Grant No. NWO 91109024], the Dutch Pink Ribbon foundation [Grant Nos. 110005 and 2014-187.WO76], BBMRI [Grant No. NWO 184.021.007/CP46] and Transcan [Grant No. JTC 2012 Cancer 12-054]. HEBON thanks the study participants and the registration teams of IKNL and PALGA for part of the data collection.
Funding
No specific external funding for this study was obtained. The Hebon study in general is supported by several grants, listed in the acknowledgements.
Author information
Authors and Affiliations
Consortia
Contributions
Conceptualization: AJ, BAMH-G, MKS, and MJH; data curation: MvB and AR; formal analysis: MvB and AR; funding acquisition: CEL, RAEMT, MGEMA, IvdB, LPVB, MdB, LPvH, CMK, MR, H, MKS, and MJH; investigation: BAMH-G, CEL, RAEMT, MGEMA, IvdB, LPVB, MdB, LPvH, CMK, MR, H, MKS, and MJH; Methodology: MvB, AR, BAMH-G, AJ, MKS, and MJH; Project Administration: BAMH-G, AJ, MKS, and MJH; Resources: CEL, RAEMT, MGEMA, IvdB, LPVB, MdB, LPvH, CMK, MR, H, MKS, and MJH; Supervision: BAMH-G, AJ, MKS, and MJH; Validation: BAMH-G, I-MO, LBK CEL, RAEMT, MGEMA, IvdB, LPVB, MdB, LPvH, CMK, MR, H, MKS, AJ, and MJH; Visualization: MvB and AR; writing—original draft: MvB and AR; writing—review and editing: MvB, AR, BAMH-G, I-MO, LBK CEL, RAEMT, MGEMA, IvdB, LPVB, MdB, LPvH, CMK, MR, H, MKS, AJ, and MJH.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to disclose.
Ethical approval
All participating centers’ Medical Ethics Committees approved the study. Written informed consent was acquired from each participating woman, or a close relative or proxy for deceased individuals. The study was conducted in accordance with the Declaration of Helsinki.
Consent for publication
No individual data are presented in this publication and individual consent therefore not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The full list of Hebon contributors is listed in the acknowledgements section.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
van Barele, M., Rieborn, A., Heemskerk-Gerritsen, B.A.M. et al. Survival of BRCA1/BRCA2-associated pT1 breast cancer patients, a cohort study. Breast Cancer Res Treat 194, 159–170 (2022). https://doi.org/10.1007/s10549-022-06608-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-022-06608-1