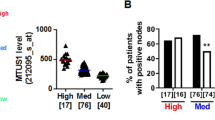
Abstract
Purpose
Paclitaxel, belongs to tubulin-binding agents (TBAs), shows a great efficacy against breast cancer via stabilizing microtubules. Drug resistance limits its clinical application. Here we aimed to explore a role of Polarity protein Par3 in improving paclitaxel effectiveness.
Methods
Breast cancer specimens from 45 patients were collected to study the relationship between Par3 expression and paclitaxel efficacy. The Kaplan–Meier method was used for survival analysis. Cell viability was measured in breast cancer cells (SK-BR-3 and T-47D) with Par3 over-expression or knockdown. The flow cytometry assays were performed to measure cell apoptosis and cell cycle. BrdU incorporation assay and Hoechst 33,258 staining were performed to measure cell proliferation and cell apoptosis, respectively. Immunofluorescence was used to detect microtubule structures.
Results
Par3 expression was associated with good response of paclitaxel in breast cancer patients. Consistently, Par3 over-expression significantly sensitized breast cancer cells to paclitaxel by promoting cell apoptosis and reducing cell proliferation. In Par3 overexpressing cells upon paclitaxel treatment, we observed intensified cell cycle arrests at metaphase. Further exploration showed that Par3 over-expression stabilized microtubules of breast cancer cells in response to paclitaxel and resists to microtubules instability induced by nocodazole, a microtubule-depolymerizing agent.
Conclusion
Par3 facilitates polymeric forms of tubulin and stabilizes microtubule structure, which aggravates paclitaxel-induced delay at the metaphase-anaphase transition, leading to proliferation inhibition and apoptosis of breast cancer cells. Par3 has a potential role in sensitizing breast cancer cells to paclitaxel, which may provide a more precise assessment of individual treatment and novel therapeutic targets.




Similar content being viewed by others
Data availability
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
References
Ferlay J, Colombet M, Soerjomataram I et al (2019) Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer 144:1941–1953. https://doi.org/10.1002/ijc.31937
Engels FK, Sparreboom A, Mathot RA et al (2005) Potential for improvement of docetaxel-based chemotherapy: a pharmacological review. Br J Cancer 93:173–177. https://doi.org/10.1038/sj.bjc.6602698
Kavallaris M (2010) Microtubules and resistance to tubulin-binding agents. Nat Rev Cancer 10:194–204. https://doi.org/10.1038/nrc2803
Alli E, Bash-Babula J, Yang JM et al (2002) Effect of stathmin on the sensitivity to antimicrotubule drugs in human breast cancer. Cancer Res 62:6864–6869
Wang Y, Yin S, Blade K et al (2006) Mutations at leucine 215 of beta-tubulin affect paclitaxel sensitivity by two distinct mechanisms. Biochemistry 45:185–194. https://doi.org/10.1021/bi051207d
Mozzetti S, Ferlini C, Concolino P et al (2005) Class III beta-tubulin overexpression is a prominent mechanism of paclitaxel resistance in ovarian cancer patients. Clin Cancer Res 11:298–305
Ahmed AA, Mills AD, Ibrahim AE et al (2007) The extracellular matrix protein TGFBI induces microtubule stabilization and sensitizes ovarian cancers to paclitaxel. Cancer Cell 12:514–527. https://doi.org/10.1016/j.ccr.2007.11.014
Chen S, Chen J, Shi H et al (2013) Regulation of microtubule stability and organization by mammalian Par3 in specifying neuronal polarity. Dev Cell 24:26–40. https://doi.org/10.1016/j.devcel.2012.11.014
Iden S, Collard JG (2008) Crosstalk between small GTPases and polarity proteins in cell polarization. Nat Rev Mol Cell Biol 9:846–859. https://doi.org/10.1038/nrm2521
Bose R, Wrana JL (2006) Regulation of Par6 by extracellular signals. Curr Opin Cell Biol 18:206–212. https://doi.org/10.1016/j.ceb.2006.02.005
Etienne-Manneville S, Hall A (2001) Integrin-mediated activation of Cdc42 controls cell polarity in migrating astrocytes through PKCzeta. Cell 106:489–498. https://doi.org/10.1016/s0092-8674(01)00471-8
Etienne-Manneville S, Hall A (2003) Cdc42 regulates GSK-3beta and adenomatous polyposis coli to control cell polarity. Nature 421:753–756. https://doi.org/10.1038/nature01423
Chen X, Macara IG (2005) Par-3 controls tight junction assembly through the Rac exchange factor Tiam1. Nat Cell Biol 7:262–269. https://doi.org/10.1038/ncb1226
Wang S, Watanabe T, Matsuzawa K et al (2012) Tiam1 interaction with the PAR complex promotes talin-mediated Rac1 activation during polarized cell migration. J Cell Biol 199:331–345. https://doi.org/10.1083/jcb.201202041
Schmoranzer J, Fawcett JP, Segura M et al (2009) Par3 and dynein associate to regulate local microtubule dynamics and centrosome orientation during migration. Curr Biol 19:1065–1074. https://doi.org/10.1016/j.cub.2009.05.065
Aranda V, Nolan ME, Muthuswamy SK (2008) Par complex in cancer: a regulator of normal cell polarity joins the dark side. Oncogene 27:6878–6887. https://doi.org/10.1038/onc.2008.340
Iden S, van Riel WE, Schafer R et al (2012) Tumor type-dependent function of the par3 polarity protein in skin tumorigenesis. Cancer Cell 22:389–403. https://doi.org/10.1016/j.ccr.2012.08.004
den Elzen N, Pines J (2001) Cyclin A is destroyed in prometaphase and can delay chromosome alignment and anaphase. J Cell Biol 153:121–136. https://doi.org/10.1083/jcb.153.1.121
Geley S, Kramer E, Gieffers C et al (2001) Anaphase-promoting complex/cyclosome-dependent proteolysis of human cyclin A starts at the beginning of mitosis and is not subject to the spindle assembly checkpoint. J Cell Biol 153:137–148. https://doi.org/10.1083/jcb.153.1.137
Etemad-Moghadam B, Guo S, Kemphues KJ (1995) Asymmetrically distributed PAR-3 protein contributes to cell polarity and spindle alignment in early C. elegans embryos. Cell 83:743–752. https://doi.org/10.1016/0092-8674(95)90187-6
Suzuki A, Ohno S (2006) The PAR-aPKC system: lessons in polarity. J Cell Sci 119:979–987. https://doi.org/10.1242/jcs.02898
Ebnet K, Suzuki A, Horikoshi Y et al (2001) The cell polarity protein ASIP/PAR-3 directly associates with junctional adhesion molecule (JAM). EMBO J 20:3738–3748. https://doi.org/10.1093/emboj/20.14.3738
Takekuni K, Ikeda W, Fujito T et al (2003) Direct binding of cell polarity protein PAR-3 to cell-cell adhesion molecule nectin at neuroepithelial cells of developing mouse. J Biol Chem 278:5497–5500. https://doi.org/10.1074/jbc.C200707200
Feng W, Wu H, Chan LN et al (2008) Par-3-mediated junctional localization of the lipid phosphatase PTEN is required for cell polarity establishment. J Biol Chem 283:23440–23449. https://doi.org/10.1074/jbc.M802482200
Nagai-Tamai Y, Mizuno K, Hirose T et al (2002) Regulated protein-protein interaction between aPKC and PAR-3 plays an essential role in the polarization of epithelial cells. Genes Cells 7:1161–1171. https://doi.org/10.1046/j.1365-2443.2002.00590.x
Wu H, Feng W, Chen J et al (2007) PDZ domains of Par-3 as potential phosphoinositide signaling integrators. Mol Cell 28:886–898. https://doi.org/10.1016/j.molcel.2007.10.028
Kunda P, Paglini G, Quiroga S et al (2001) Evidence for the involvement of Tiam1 in axon formation. J Neurosci 21:2361–2372
Nishimura T, Yamaguchi T, Kato K et al (2005) PAR-6-PAR-3 mediates Cdc42-induced Rac activation through the Rac GEFs STEF/Tiam1. Nat Cell Biol 7:270–277. https://doi.org/10.1038/ncb1227
McCaffrey LM, Montalbano J, Mihai C et al (2012) Loss of the Par3 polarity protein promotes breast tumorigenesis and metastasis. Cancer Cell 22:601–614. https://doi.org/10.1016/j.ccr.2012.10.003
Xue B, Krishnamurthy K, Allred DC et al (2013) Loss of Par3 promotes breast cancer metastasis by compromising cell-cell cohesion. Nat Cell Biol 15:189–200. https://doi.org/10.1038/ncb2663
Wang S, Cai J, Zhang S et al (2021) Loss of polarity protein Par3, via transcription factor Snail, promotes bladder cancer metastasis. Cancer Sci 112:2625–2641. https://doi.org/10.1111/cas.14920
Jordan MA, Wilson L (2004) Microtubules as a target for anticancer drugs. Nat Rev Cancer 4:253–265. https://doi.org/10.1038/nrc1317
Rieder CL, Maiato H (2004) Stuck in division or passing through: what happens when cells cannot satisfy the spindle assembly checkpoint. Dev Cell 7:637–651. https://doi.org/10.1016/j.devcel.2004.09.002
Zhang H, Macara IG (2006) The polarity protein PAR-3 and TIAM1 cooperate in dendritic spine morphogenesis. Nat Cell Biol 8:227–237. https://doi.org/10.1038/ncb1368
Marupudi NI, Han JE, Li KW et al (2007) Paclitaxel: a review of adverse toxicities and novel delivery strategies. Expert Opin Drug Saf 6:609–621. https://doi.org/10.1517/14740338.6.5.609
Zhang D, Yang R, Wang S et al (2014) Paclitaxel: new uses for an old drug. Drug Des Devel Ther 8:279–284. https://doi.org/10.2147/DDDT.S56801
Walker FE (1993) Paclitaxel (TAXOL): side effects and patient education issues. Semin Oncol Nurs 9:6–10. https://doi.org/10.1016/s0749-2081(16)30036-5
Zhao Y, Yao D, Li Y et al (2021) Loss of polarity protein Par3 is mediated by transcription factor Sp1 in breast cancer. Biochem Biophys Res Commun 561:172–179. https://doi.org/10.1016/j.bbrc.2021.05.025
Funding
This work was supported by the National Natural Science Foundation (Grant Nos. 81772615, 81972294, 81772968) and Shanghai Anticancer Association (SACA-CY20B02).
Author information
Authors and Affiliations
Contributions
SC, YX and BW designed the study. YZ, HP, LL, YL, XH collected the clinical data, performed the experiments, created the figures and tables. YZ, YX and SC wrote and edited the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
All authors declared that they had no conflicts of interest.
Ethical approval
This study was approved by the research ethical committee of Fudan University Shanghai Cancer Center.
Consent to participate
The consent was obtained from each patient before clinical data analyses.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhao, Y., Peng, H., Liang, L. et al. Polarity protein Par3 sensitizes breast cancer to paclitaxel by promoting cell cycle arrest. Breast Cancer Res Treat 192, 75–87 (2022). https://doi.org/10.1007/s10549-021-06490-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-021-06490-3