Abstract
Purpose
To examine benefit of sulindac for relief of musculoskeletal symptoms (MSS) in patients stable on aromatase inhibitors (AIs).
Methods
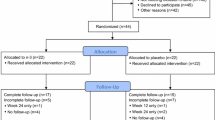
Sulindac was evaluated at 150 mg twice daily for effects on MSS at 3, 6, 9, and 12 months in 50 postmenopausal women stable on AI therapy for a median of 12.5 months for hormone receptor-positive breast cancer. A separate, non-randomized group of 50 similar patients was observed for change in MSS over 12 months. MSS severity was assessed using the Western Ontario and McMaster Universities Osteoarthritis (WOMAC) Index and Brief Pain Inventory Short Form (BPI-SF). The Functional Assessment of Cancer Therapy—General form (FACT-G) measured quality of life (QOL). Change in MSS and QOL across time was assessed in each group using linear mixed effects models.
Results
Stiffness, not pain, was the main complaint at baseline. At 12 months, sulindac patients reported decreases (improvements) in mean (95% CI) Total WOMAC score [− 5.85 (− 9.73, − 1.96)] and WOMAC pain [− 5.40 (− 10.64, − 0 .18)], Stiffness [− 9.53 (− 14.98, − 4.08)] and Physical Function [− 5.61 (− 9.62, − 1.60)] subscales, but not BPI-SF worst pain. Among sulindac patients with higher baseline MSS severity, 35% experienced ≥ 50% improvement in Total WOMAC and Total FACT-G scores [6.18 (2.08, 10.27); P = 0.003]. For the observation group, MSS and QOL did not improve over 12 months, even among those with higher baseline MSS severity.
Conclusions
Sulindac may relieve MSS in AI patients, especially physical function and stiffness. Randomized controlled trials should further evaluate NSAIDs on AI-MSS and AI adherence.
Trial registration number and date of registration
NCT01761877, December, 2012.

Similar content being viewed by others
Data availability
All associated data are available in a data repository and are available upon request.
Code availability
Not Applicable.
References
Early Breast Cancer Trialists’ Collaborative G (2015) Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet 386:1341–1352. https://doi.org/10.1016/S0140-6736(15)61074-1
Chirgwin JH, Giobbie-Hurder A, Coates AS, Price KN, Ejlertsen B, Debled M, Gelber RD, Goldhirsch A, Smith I, Rabaglio M, Forbes JF, Neven P, Lang I, Colleoni M, Thurlimann B (2016) Treatment adherence and its impact on disease-free survival in the breast international group 1–98 trial of tamoxifen and letrozole, alone and in sequence. J Clin Oncol 34:2452–2459. https://doi.org/10.1200/JCO.2015.63.8619
Chlebowski RT, Kim J, Haque R (2014) Adherence to endocrine therapy in breast cancer adjuvant and prevention settings. Cancer Prev Res (Phila) 7:378–387. https://doi.org/10.1158/1940-6207.CAPR-13-0389
Hershman DL, Shao T, Kushi LH, Buono D, Tsai WY, Fehrenbacher L, Kwan M, Gomez SL, Neugut AI (2011) Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast Cancer Res Treat 126:529–537. https://doi.org/10.1007/s10549-010-1132-4
Murphy CC, Bartholomew LK, Carpentier MY, Bluethmann SM, Vernon SW (2012) Adherence to adjuvant hormonal therapy among breast cancer survivors in clinical practice: a systematic review. Breast Cancer Res Treat 134:459–478. https://doi.org/10.1007/s10549-012-2114-5
Partridge AH, LaFountain A, Mayer E, Taylor BS, Winer E, Asnis-Alibozek A (2008) Adherence to initial adjuvant anastrozole therapy among women with early-stage breast cancer. J Clin Oncol 26:556–562. https://doi.org/10.1200/JCO.2007.11.5451
van Hellemond IEG, Geurts SME, Tjan-Heijnen VCG (2018) Current status of extended adjuvant endocrine therapy in early stage breast cancer. Curr Treat Options Oncol 19:26. https://doi.org/10.1007/s11864-018-0541-1
Hershman DL, Kushi LH, Shao T, Buono D, Kershenbaum A, Tsai W-Y, Fehrenbacher L, Lin Gomez S, Miles S, Neugut AI (2010) Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients. J Clin Oncol 28:4120–4128. https://doi.org/10.1200/JCO.2009.25.9655
Condorelli R, Vaz-Luis I (2018) Managing side effects in adjuvant endocrine therapy for breast cancer. Expert Rev Anticancer Ther 18:1101–1112. https://doi.org/10.1080/14737140.2018.1520096
Kwan ML, Roh JM, Laurent CA, Lee J, Tang L, Hershman D, Kushi LH, Yao S (2017) Patterns and reasons for switching classes of hormonal therapy among women with early-stage breast cancer. Cancer Causes Control 28:557–562. https://doi.org/10.1007/s10552-017-0888-9
Crew KD, Greenlee H, Capodice J, Raptis G, Brafman L, Fuentes D, Sierra A, Hershman DL (2007) Prevalence of joint symptoms in postmenopausal women taking aromatase inhibitors for early-stage breast cancer. J Clin Oncol 25:3877–3883. https://doi.org/10.1200/JCO.2007.10.7573
Beckwée D, Leysen L, Meuwis K, Adriaenssens N (2017) Prevalence of aromatase inhibitor-induced arthralgia in breast cancer: a systematic review and meta-analysis. Support Care Cancer 25:1673–1686. https://doi.org/10.1007/s00520-017-3613-z
Henry NL, Giles JT, Ang D, Mohan M, Dadabhoy D, Robarge J, Hayden J, Lemler S, Shahverdi K, Powers P, Li L, Flockhart D, Stearns V, Hayes DF, Storniolo AM, Clauw DJ (2008) Prospective characterization of musculoskeletal symptoms in early stage breast cancer patients treated with aromatase inhibitors. Breast Cancer Res Treat 111:365–372. https://doi.org/10.1007/s10549-007-9774-6
Howell A, Cuzick J, Baum M, Buzdar A, Dowsett M, Forbes JF, Hoctin-Boes G, Houghton J, Locker GY, Tobias JS, ATAC Trialists’ Group (2005) Results of the ATAC (arimidex, tamoxifen, alone or in combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. The Lancet 365:60–62. https://doi.org/10.1016/S0140-6736(04)17666-6
Laroche F, Coste J, Medkour T, Cottu PH, Pierga J-Y, Lotz J-P, Beerblock K, Tournigand C, Declèves X, de Cremoux P, Bouhassira D, Perrot S (2014) Classification of and risk factors for estrogen deprivation pain syndromes related to aromatase inhibitor treatments in women with breast cancer: a prospective multicenter cohort study. J Pain 15:293–303. https://doi.org/10.1016/j.jpain.2013.11.004
Borrie AE, Kim RB (2017) Molecular basis of aromatase inhibitor associated arthralgia: known and potential candidate genes and associated biomarkers. Expert Opin Drug Metab Toxicol 13:149–156. https://doi.org/10.1080/17425255.2017.1234605
Robarge JD, Duarte DB, Shariati B, Wang R, Flockhart DA, Vasko MR (2016) Aromatase inhibitors augment nociceptive behaviors in rats and enhance the excitability of sensory neurons. Exp Neurol 281:53–65. https://doi.org/10.1016/j.expneurol.2016.04.006
Fusi C, Materazzi S, Benemei S, Coppi E, Trevisan G, Marone IM, Minocci D, De Logu F, Tuccinardi T, Di Tommaso MR, Susini T, Moneti G, Pieraccini G, Geppetti P, Nassini R (2014) Steroidal and non-steroidal third-generation aromatase inhibitors induce pain-like symptoms via TRPA1. Nat Commun 5:5736. https://doi.org/10.1038/ncomms6736
De Logu F, Tonello R, Materazzi S, Nassini R, Fusi C, Coppi E, Li Puma S, Marone IM, Sadofsky LR, Morice AH, Susini T, Terreni A, Moneti G, Di Tommaso M, Geppetti P, Benemei S (2016) TRPA1 mediates aromatase inhibitor-evoked pain by the aromatase substrate androstenedione. J Cancer Res 76:7024–7035. https://doi.org/10.1158/0008-5472.CAN-16-1492
Henry NL, Unger JM, Schott AF, Fehrenbacher L, Flynn PJ, Prow DM, Sharer CW, Burton GV, Kuzma CS, Moseley A, Lew DL, Fisch MJ, Moinpour CM, Hershman DL, Wade JL (2017) Randomized, multicenter, placebo-controlled clinical trial of duloxetine versus placebo for aromatase inhibitor-associated arthralgias in early-stage breast cancer: SWOG S1202. J Clin Oncol 36:326–332. https://doi.org/10.1200/JCO.2017.74.6651
Hershman DL, Unger JM, Greenlee H, Capodice JL, Lew DL, Darke AK, Kengla AT, Melnik MK, Jorgensen CW, Kreisle WH, Minasian LM, Fisch MJ, Henry NL, Crew KD (2018) Effect of acupuncture vs sham acupuncture or waitlist control on joint pain related to aromatase inhibitors among women with early-stage breast cancer: a randomized clinical trial. JAMA 320:167–176. https://doi.org/10.1001/jama.2018.8907
Chen L, Lin CC, Huang TW, Kuan YC, Huang YH, Chen HC, Kao CY, Su CM, Tam KW (2017) Effect of acupuncture on aromatase inhibitor-induced arthralgia in patients with breast cancer: a meta-analysis of randomized controlled trials. Breast 33:132–138. https://doi.org/10.1016/j.breast.2017.03.015
Gupta A, Henry NL, Loprinzi CL (2020) Management of aromatase inhibitor-induced musculoskeletal symptoms. JCO Oncol Pract 16:733–739. https://doi.org/10.1200/OP.20.00113
Niravath P (2013) Aromatase inhibitor-induced arthralgia: a review. Ann Oncol 24:1443–1449. https://doi.org/10.1093/annonc/mdt037
Lu G, Zheng J, Zhang L (2020) The effect of exercise on aromatase inhibitor-induced musculoskeletal symptoms in breast cancer survivors :a systematic review and meta-analysis. Support Care Cancer 28:1587–1596. https://doi.org/10.1007/s00520-019-05186-1
Thompson PA, Huang C, Yang J, Wertheim BC, Roe DJ, Zhang X, Ding J, Chalasani P, Preece C, Martinez JA, Chow H-H, Sherry, Stopeck AT (2021) Evidence that the non-selective NSAID sulindac reduces breast density in postmenopausal women on aromatase inhibitors. Clinical Cancer Research in press.
Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW (1988) Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol 15:1833–1840
Cleeland CS, Ryan KM (1994) Pain assessment: global use of the brief pain inventory. Ann Acad Med Singap 23:129–138
Mendoza T, Mayne T, Rublee D, Cleeland C (2006) Reliability and validity of a modified Brief Pain Inventory short form in patients with osteoarthritis. Eur J Pain 10:353–353. https://doi.org/10.1016/j.ejpain.2005.06.002
Fairclough DL, Cella DF (1996) Functional assessment of cancer therapy (FACT-G): non-response to individual questions. Qual Life Res 5:321–329. https://doi.org/10.1007/bf00433916
Webster K, Cella D, Yost K (2003) The functional assessment of chronic illness therapy (FACIT) measurement system: properties, applications, and interpretation. Health Qual Life Outcomes 1:79. https://doi.org/10.1186/1477-7525-1-79
Tubach F, Ravaud P, Beaton D, Boers M, Bombardier C, Felson DT, van der Heijde D, Wells G, Dougados M (2007) Minimal clinically important improvement and patient acceptable symptom state for subjective outcome measures in rheumatic disorders. J Rheumatol 34:1188
Shapiro AC, Adlis SA, Robien K, Kirstein MN, Liang S, Richter SA, Lerner RE (2016) Randomized, blinded trial of vitamin D3 for treating aromatase inhibitor-associated musculoskeletal symptoms (AIMSS). Breast Cancer Res Treat 155:501–512. https://doi.org/10.1007/s10549-016-3710-6
Niravath P, Hilsenbeck SG, Wang T, Jiralerspong S, Nangia J, Pavlick A, Ademuyiwa F, Frith A, Ma C, Park H, Rigden C, Suresh R, Ellis M, Kent Osborne C, Rimawi MF (2019) Randomized controlled trial of high-dose versus standard-dose vitamin D3 for prevention of aromatase inhibitor-induced arthralgia. Breast Cancer Res Treat 177:427–435. https://doi.org/10.1007/s10549-019-05319-4
Hershman DL, Unger JM, Crew KD, Awad D, Dakhil SR, Gralow J, Greenlee H, Lew DL, Minasian LM, Till C, Wade JL, Meyskens FL, Moinpour CM (2015) Randomized multicenter placebo-controlled trial of omega-3 fatty acids for the control of aromatase inhibitor-induced musculoskeletal pain: SWOG S0927. J Clin Oncol 33:1910–1917. https://doi.org/10.1200/JCO.2014.59.5595
Lustberg MB, Orchard TS, Reinbolt R, Andridge R, Pan X, Belury M, Cole R, Logan A, Layman R, Ramaswamy B, Wesolowski R, Berger M, Patterson E, Loprinzi C, Shapiro CL, Yee L (2018) Randomized placebo-controlled pilot trial of omega 3 fatty acids for prevention of aromatase inhibitor-induced musculoskeletal pain. Breast Cancer Res Treat 167:709–718. https://doi.org/10.1007/s10549-017-4559-z
Ringash J, O’Sullivan B, Bezjak A, Redelmeier DA (2007) Interpreting clinically significant changes in patient-reported outcomes. Cancer 110:196–202. https://doi.org/10.1002/cncr.22799
Chow LWC, Yip AYS, Chu WP, Loo WTY, Toi M (2011) Bone metabolism and quality-of-life of postmenopausal women with invasive breast cancer receiving neoadjuvant hormonal therapy: Sub-analyses from celecoxib anti-aromatase neoadjuvant (CAAN) trial. J Steroid Biochem Mol Biol 125:112–119. https://doi.org/10.1016/j.jsbmb.2010.12.018
Rosati MS, Di Seri M, Baciarello G, Lo Russo V, Grassi P, Marchetti L, Giovannoni S, Basile ML, Frati L (2011) Etoricoxib and anastrozole in adjuvant early breast cancer: ETAN trial (phase III). J Clin Oncol 29:533–533. https://doi.org/10.1200/jco.2011.29.15_suppl.533
Marcum ZA, Hanlon JT (2010) Recognizing the risks of chronic nonsteroidal anti-inflammatory drug use in older adults. Ann Longterm Care 18:24–27
Acknowledgements
None.
Funding
The work was supported by funding from the National Cancer Institute (Grant Number R01CA161534).
Author information
Authors and Affiliations
Contributions
Conception and design of study (PAT, ATS, and DJR); acquisition of data (PC, JC, and LB); analysis and/or interpretation of data (JAM, BCW, DJR, and PAT); drafting the manuscript (PAT, JAM, and BCW); approval of the version of the manuscript to be published (PAT, JAM, BCW, DJR, PC, JC, LB, HSC, and ATS).
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to disclose.
Ethical approval
The study was approved by the institutional review boards (IRB) at both the University of Arizona, Tucson, and Stony Brook University, New York.
Consent to participate
All patients provided written informed consent.
Consent for publication
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funding agencies had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Martinez, J.A., Wertheim, B.C., Roe, D.J. et al. Sulindac Improves Stiffness and Quality of Life in Women Taking Aromatase Inhibitors for Breast Cancer. Breast Cancer Res Treat 192, 113–122 (2022). https://doi.org/10.1007/s10549-021-06485-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-021-06485-0