Abstract
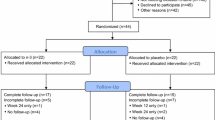
The purpose of the study was to evaluate the efficacy and safety of vitamin D3 at 4000 IU/day as a treatment option for aromatase inhibitor-associated musculoskeletal symptoms (AIMSS) when compared with the usual care dose of 600 IU D3. We conducted a single site randomized, double-blind, phase 3 clinical trial in women with AIMSS comparing change in symptoms, reproductive hormones and AI pharmacokinetics. Postmenopausal women ≥18 years with stages I–IIIA breast cancer, taking AI and experiencing AIMSS [breast cancer prevention trial symptom scale-musculoskeletal (BCPT-MS) subscale ≥1.5] were admitted. Following randomization, 116 patients had a run-in period of 1 month on 600 IU D3, then began the randomized assignment to either 600 IU D3 (n = 56) or 4000 IU D3 (n = 57) daily for 6 months. The primary endpoint was a change in AIMSS from baseline (after 1 month run-in) on the BCPT-MS (general MS pain, joint pain, muscle stiffness, range for each question: 0 = not at all to 4 = extremely). Groups had no statistically significant differences demographically or clinically. There were no discernable differences between the randomly allocated treatment groups at 6 months in measures of AIMSS, pharmacokinetics of anastrozole and letrozole, serum levels of reproductive hormones, or adverse events. We found no significant changes in AIMSS measures between women who took 4000 IU D3 daily compared with 600 IU D3. The 4000 IU D3 did not adversely affect reproductive hormone levels or the steady state pharmacokinetics of anastrozole or letrozole. In both groups, serum 25(OH)D remained in the recommended range for bone health (≥30 ng/mL) and safety (<50 ng/mL).



Similar content being viewed by others
References
Khan QJ, Kimler BF, Reddy PS, Sharma P, Klemp JR, Fabian CJ (2012) Randomized trial of vitamin D3 to prevent worsening of musculoskeletal symptoms and fatigue in women with breast cancer starting adjuvant letrozole: the VITAL trial. ASCO Meet Abstr 30(15_Suppl):9000
Rastelli AL, Taylor ME, Gao F, Armamento-Villareal R, Jamalabadi-Majidi S, Napoli N, Ellis MJ (2011) Vitamin D and aromatase inhibitor-induced musculoskeletal symptoms (AIMSS): a phase II, double-blind, placebo-controlled, randomized trial. Breast Cancer Res Treat 129(1):107–116. doi:10.1007/s10549-011-1644-6
Prieto-Alhambra D, Javaid MK, Servitja S, Arden NK, Martinez-Garcia M, Diez-Perez A, Albanell J, Tusquets I, Nogues X (2011) Vitamin D threshold to prevent aromatase inhibitor-induced arthralgia: a prospective cohort study. Breast Cancer Res Treat 125(3):869–878. doi:10.1007/s10549-010-1075-9
Singer O, Cigler T, Moore AB, Levine AB, Do HT, Mandl LA (2014) Hypovitaminosis D is a predictor of aromatase inhibitor musculoskeletal symptoms. Breast J 20(2):174–179. doi:10.1111/tbj.12227
Khan QJ, Reddy PS, Kimler BF, Sharma P, Baxa SE, O’Dea AP, Klemp JR, Fabian CJ (2010) Effect of vitamin D supplementation on serum 25-hydroxy vitamin D levels, joint pain, and fatigue in women starting adjuvant letrozole treatment for breast cancer. Breast Cancer Res Treat 119(1):111–118. doi:10.1007/s10549-009-0495-x
Waltman NL, Ott CD, Twiss JJ, Gross GJ, Lindsey AM (2009) Vitamin D insufficiency and musculoskeletal symptoms in breast cancer survivors on aromatase inhibitor therapy. Cancer Nurs 32(2):143–150. doi:10.1097/01.NCC.0000339262.44560.92
Lintermans A, Laenen A, Van Calster B, Van Hoydonck M, Pans S, Verhaeghe J, Westhovens R, Henry NL, Wildiers H, Paridaens R, Dieudonne AS, Leunen K, Morales L, Verschueren K, Timmerman D, De Smet L, Vergote I, Christiaens MR, Neven P (2013) Prospective study to assess fluid accumulation and tenosynovial changes in the aromatase inhibitor-induced musculoskeletal syndrome: 2-year follow-up data. Ann Oncol Off J Eur Soc Med Oncol 24(2):350–355. doi:10.1093/annonc/mds290
Niravath P (2013) Aromatase inhibitor-induced arthralgia: a review. Ann Oncol Off J Eur Soc Med Oncol 24(6):1443–1449. doi:10.1093/annonc/mdt037
Mao JJ, Stricker C, Bruner D, Xie S, Bowman MA, Farrar JT, Greene BT, DeMichele A (2009) Patterns and risk factors associated with aromatase inhibitor-related arthralgia among breast cancer survivors. Cancer 115(16):3631–3639. doi:10.1002/cncr.24419
Henry NL, Giles JT, Stearns V (2008) Aromatase inhibitor-associated musculoskeletal symptoms: etiology and strategies for management. Oncology (Williston Park) 22(12):1401–1408
Crew KD, Greenlee H, Capodice J, Raptis G, Brafman L, Fuentes D, Sierra A, Hershman DL (2007) Prevalence of joint symptoms in postmenopausal women taking aromatase inhibitors for early-stage breast cancer. J Clin Oncol Off J Am Soc Clin Oncol 25(25):3877–3883. doi:10.1200/JCO.2007.10.7573
Morales L, Pans S, Verschueren K, Van Calster B, Paridaens R, Westhovens R, Timmerman D, De Smet L, Vergote I, Christiaens MR, Neven P (2008) Prospective study to assess short-term intra-articular and tenosynovial changes in the aromatase inhibitor-associated arthralgia syndrome. J Clin Oncol Off J Am Soc Clin Oncol 26(19):3147–3152. doi:10.1200/JCO.2007.15.4005
Fusi C, Materazzi S, Benemei S, Coppi E, Trevisan G, Marone IM, Minocci D, De Logu F, Tuccinardi T, Di Tommaso MR, Susini T, Moneti G, Pieraccini G, Geppetti P, Nassini R (2014) Steroidal and non-steroidal third-generation aromatase inhibitors induce pain-like symptoms via TRPA1. Nat Commun 5:5736. doi:10.1038/ncomms6736
Holick MF (2006) High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc 81(3):353–373. doi:10.4065/81.3.353
Glerup H, Mikkelsen K, Poulsen L, Hass E, Overbeck S, Andersen H, Charles P, Eriksen EF (2000) Hypovitaminosis D myopathy without biochemical signs of osteomalacic bone involvement. Calcif Tissue Int 66(6):419–424
Holick MF (2007) Vitamin D deficiency. N Engl J Med 357(3):266–281. doi:10.1056/NEJMra070553
Felson DT, Cummings SR (2005) Aromatase inhibitors and the syndrome of arthralgias with estrogen deprivation. Arthritis Rheum 52(9):2594–2598. doi:10.1002/art.21364
Ceglia L (2008) Vitamin D and skeletal muscle tissue and function. Mol Asp Med 29(6):407–414. doi:10.1016/j.mam.2008.07.002
Boonen S, Bischoff-Ferrari HA, Cooper C, Lips P, Ljunggren O, Meunier PJ, Reginster JY (2006) Addressing the musculoskeletal components of fracture risk with calcium and vitamin D: a review of the evidence. Calcif Tissue Int 78(5):257–270. doi:10.1007/s00223-005-0009-8
National Research Council (2011) Dietary reference intakes for calcium and vitamin D. The National Academies Press, Washington, DC
Gralow JR, Biermann JS, Farooki A, Fornier MN, Gagel RF, Kumar R, Litsas G, McKay R, Podoloff DA, Srinivas S, Van Poznak CH (2013) NCCN Task Force report: bone health in cancer care. J Natl Compr Cancer Netw 11(Suppl 3):S-1–S-50
Kristal AR, Darke AK, Morris JS, Tangen CM, Goodman PJ, Thompson IM, Meyskens FL Jr, Goodman GE, Minasian LM, Parnes HL, Lippman SM, Klein EA (2014) Baseline selenium status and effects of selenium and vitamin E supplementation on prostate cancer risk. J Natl Cancer Inst 106(3):djt456. doi:10.1093/jnci/djt456
Greenwald P, Anderson D, Nelson SA, Taylor PR (2007) Clinical trials of vitamin and mineral supplements for cancer prevention. Am J Clin Nutr 85(1):314S–317S
Goodwin PJ, Ennis M, Pritchard KI, Koo J, Hood N (2009) Prognostic effects of 25-hydroxyvitamin D levels in early breast cancer. J Clin Oncol Off J Am Soc Clin Oncol 27(23):3757–3763. doi:10.1200/JCO.2008.20.0725
Kristal AR, Till C, Song X, Tangen CM, Goodman PJ, Neuhauser ML, Schenk JM, Thompson IM, Meyskens FL Jr, Goodman GE, Minasian LM, Parnes HL, Klein EA (2014) Plasma vitamin D and prostate cancer risk: results from the Selenium and Vitamin E Cancer Prevention Trial. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cospons Am Soc Prev Oncol 23(8):1494–1504. doi:10.1158/1055-9965.EPI-14-0115
Manson JE, Bassuk SS (2015) Vitamin D research and clinical practice: at a crossroads. JAMA 313(13):1311–1312. doi:10.1001/jama.2015.1353
Swenson KK, Nissen MJ, Henly SJ, Maybon L, Pupkes J, Zwicky K, Tsai ML, Shapiro AC (2013) Identification of tools to measure changes in musculoskeletal symptoms and physical functioning in women with breast cancer receiving aromatase inhibitors. Oncol Nurs Forum 40(6):549–557. doi:10.1188/13.ONF.549-557
World Health Organization FRAX® WHO fracture risk assessment tool. World Health Organization Collaborating Centre for Metabolic Bone Diseases, University of Sheffield. http://www.sheffield.ac.uk/FRAX/. Accessed 13 Oct 2015
Bellamy N, Sothern RB, Campbell J, Buchanan WW (2002) Rhythmic variations in pain, stiffness, and manual dexterity in hand osteoarthritis. Ann Rheum Dis 61(12):1075–1080
Allen KD, DeVellis RF, Renner JB, Kraus VB, Jordan JM (2007) Validity and factor structure of the AUSCAN Osteoarthritis Hand Index in a community-based sample. Osteoarthr Cartil/Osteoarthr Res Soc 15(7):830–836. doi:10.1016/j.joca.2007.01.012
Bellamy N (2005) The WOMAC Knee and Hip Osteoarthritis Indices: development, validation, globalization and influence on the development of the AUSCAN Hand Osteoarthritis Indices. Clin Exp Rheumatol 23(5 Suppl 39):S148–S153
Stanton AL, Bernaards CA, Ganz PA (2005) The BCPT symptom scales: a measure of physical symptoms for women diagnosed with or at risk for breast cancer. J Natl Cancer Inst 97(6):448–456. doi:10.1093/jnci/dji069
Fries JF, Cella D, Rose M, Krishnan E, Bruce B (2009) Progress in assessing physical function in arthritis: PROMIS short forms and computerized adaptive testing. J Rheumatol 36(9):2061–2066. doi:10.3899/jrheum.090358
Mathiowetz V, Kashman N, Volland G, Weber K, Dowe M, Rogers S (1985) Grip and pinch strength: normative data for adults. Arch Phys Med Rehabil 66(2):69–74
Apostolou C, Dotsikas Y, Kousoulos C, Loukas YL (2008) Development and validation of an improved high-throughput method for the determination of anastrozole in human plasma by LC–MS/MS and atmospheric pressure chemical ionization. J Pharm Biomed Anal 48(3):853–859. doi:10.1016/j.jpba.2008.06.006
Goebelsmann U, Bernstein GS, Gale JA, Kletzky OA, Nakamure RM, Coulson AH, Korelitz JJ (1979) Serum gonadotropin, testosterone, estradiol and estrone levels prior to and following bilateral vasectomy. In: Lepow IH, Crozier R (eds) Vasectomy: immunologic and pathophysiologic effects in animals and man. Academic Press, New York, pp 165–181
Probst-Hensch NM, Ingles SA, Diep AT, Haile RW, Stanczyk FZ, Kolonel LN, Henderson BE (1999) Aromatase and breast cancer susceptibility. Endocr Relat Cancer 6(2):165–173
Rinaldi S, Geay A, Dechaud H, Biessy C, Zeleniuch-Jacquotte A, Akhmedkhanov A, Shore RE, Riboli E, Toniolo P, Kaaks R (2002) Validity of free testosterone and free estradiol determinations in serum samples from postmenopausal women by theoretical calculations. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cospons Am Soc Prev Oncol 11(10 Pt 1):1065–1071
Sodergard R, Backstrom T, Shanbhag V, Carstensen H (1982) Calculation of free and bound fractions of testosterone and estradiol-17 beta to human plasma proteins at body temperature. J Steroid Biochem 16(6):801–810
Vermeulen A, Verdonck L, Kaufman JM (1999) A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab 84(10):3666–3672. doi:10.1210/jcem.84.10.6079
Glanz K, Yaroch AL, Dancel M, Saraiya M, Crane LA, Buller DB, Manne S, O’Riordan DL, Heckman CJ, Hay J, Robinson JK (2008) Measures of sun exposure and sun protection practices for behavioral and epidemiologic research. Arch Dermatol 144(2):217–222. doi:10.1001/archdermatol.2007.46
Ainsworth BE, Haskell WL, Leon AS, Jacobs DR Jr, Montoye HJ, Sallis JF, Paffenbarger RS Jr (1993) Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc 25(1):71–80
Rowland M, Tozer TN, Rowland M (2011) Clinical pharmacokinetics and pharmacodynamics: concepts and applications, 4th edn. Wolters Kluwer Health/Lippincott William and Wilkins, Philadelphia
Helzlsouer KJ, Gallicchio L, MacDonald R, Wood B, Rushovich E (2012) A prospective study of aromatase inhibitor therapy, vitamin D, C-reactive protein and musculoskeletal symptoms. Breast Cancer Res Treat 131(1):277–285. doi:10.1007/s10549-011-1729-2
Singh S, Cuzick J, Mesher D, Richmond B, Howell A (2012) Effect of baseline serum vitamin D levels on aromatase inhibitors induced musculoskeletal symptoms: results from the IBIS-II, chemoprevention study using anastrozole. Breast Cancer Res Treat 132(2):625–629. doi:10.1007/s10549-011-1911-6
Lintermans A, Van Asten K, Wildiers H, Laenen A, Paridaens R, Weltens C, Verhaeghe J, Vanderschueren D, Smeets A, Van Limbergen E, Leunen K, Christiaens MR, Neven P (2014) A prospective assessment of musculoskeletal toxicity and loss of grip strength in breast cancer patients receiving adjuvant aromatase inhibitors and tamoxifen, and relation with BMI. Breast Cancer Res Treat 146(1):109–116. doi:10.1007/s10549-014-2986-7
Visser M, Deeg DJ, Lips P (2003) Low vitamin D and high parathyroid hormone levels as determinants of loss of muscle strength and muscle mass (sarcopenia): the Longitudinal Aging Study Amsterdam. J Clin Endocrinol Metab 88(12):5766–5772. doi:10.1210/jc.2003-030604
Enjuanes A, Garcia-Giralt N, Supervia A, Nogues X, Mellibovsky L, Carbonell J, Grinberg D, Balcells S, Diez-Perez A (2003) Regulation of CYP19 gene expression in primary human osteoblasts: effects of vitamin D and other treatments. Eur J Endocrinol/Eur Fed Endocr Soc 148(5):519–526
Enjuanes A, Garcia-Giralt N, Supervia A, Nogues X, Ruiz-Gaspa S, Bustamante M, Mellibovsky L, Grinberg D, Balcells S, Diez-Perez A (2005) Functional analysis of the I.3, I.6, pII and I.4 promoters of CYP19 (aromatase) gene in human osteoblasts and their role in vitamin D and dexamethasone stimulation. Eur J Endocrinol/Eur Fed Endocr Soc 153(6):981–988. doi:10.1530/eje.1.02032
Lou YR, Murtola T, Tuohimaa P (2005) Regulation of aromatase and 5alpha-reductase by 25-hydroxyvitamin D(3), 1alpha,25-dihydroxyvitamin D(3), dexamethasone and progesterone in prostate cancer cells. J Steroid Biochem Mol Biol 94(1–3):151–157. doi:10.1016/j.jsbmb.2005.01.024
Pino AM, Rodriguez JM, Rios S, Astudillo P, Leiva L, Seitz G, Fernandez M, Rodriguez JP (2006) Aromatase activity of human mesenchymal stem cells is stimulated by early differentiation, vitamin D and leptin. J Endocrinol 191(3):715–725. doi:10.1677/joe.1.07026
Barrera D, Avila E, Hernandez G, Halhali A, Biruete B, Larrea F, Diaz L (2007) Estradiol and progesterone synthesis in human placenta is stimulated by calcitriol. J Steroid Biochem Mol Biol 103(3–5):529–532. doi:10.1016/j.jsbmb.2006.12.097
Yague JG, Garcia-Segura LM, Azcoitia I (2009) Selective transcriptional regulation of aromatase gene by vitamin D, dexamethasone, and mifepristone in human glioma cells. Endocrine 35(2):252–261. doi:10.1007/s12020-008-9134-2
Cescon DW, Ennis M, Ganz PA, Beddows S, Stanczyk FZ, Sridhar SS, Goodwin PJ (2011) An analysis of vitamin D (Vit D) and serum estrogens in postmenopausal (PM) breast cancer (BC) patients receiving aromatase inhibitors (AIs). ASCO Meet Abstr 29(15_Suppl):596
Irwin ML, Cartmel B, Gross CP, Ercolano E, Li F, Yao X, Fiellin M, Capozza S, Rothbard M, Zhou Y, Harrigan M, Sanft T, Schmitz K, Neogi T, Hershman D, Ligibel J (2015) Randomized exercise trial of aromatase inhibitor-induced arthralgia in breast cancer survivors. J Clin Oncol Off J Am Soc Clin Oncol 33(10):1104–1111. doi:10.1200/JCO.2014.57.1547
Galantino ML, Callens ML, Cardena GJ, Piela NL, Mao JJ (2013) Tai chi for well-being of breast cancer survivors with aromatase inhibitor-associated arthralgias: a feasibility study. Altern Ther Health Med 19(6):38–44
Galantino ML, Desai K, Greene L, Demichele A, Stricker CT, Mao JJ (2012) Impact of yoga on functional outcomes in breast cancer survivors with aromatase inhibitor-associated arthralgias. Integr Cancer Ther 11(4):313–320. doi:10.1177/1534735411413270
Greenlee H, Crew KD, Shao T, Kranwinkel G, Kalinsky K, Maurer M, Brafman L, Insel B, Tsai WY, Hershman DL (2013) Phase II study of glucosamine with chondroitin on aromatase inhibitor-associated joint symptoms in women with breast cancer. Support Care Cancer 21(4):1077–1087. doi:10.1007/s00520-012-1628-z
Crew KD, Capodice JL, Greenlee H, Brafman L, Fuentes D, Awad D, Yann Tsai W, Hershman DL (2010) Randomized, blinded, sham-controlled trial of acupuncture for the management of aromatase inhibitor-associated joint symptoms in women with early-stage breast cancer. J Clin Oncol Off J Am Soc Clin Oncol 28(7):1154–1160. doi:10.1200/JCO.2009.23.4708
Bao T, Cai L, Giles JT, Gould J, Tarpinian K, Betts K, Medeiros M, Jeter S, Tait N, Chumsri S, Armstrong DK, Tan M, Folkerd E, Dowsett M, Singh H, Tkaczuk K, Stearns V (2013) A dual-center randomized controlled double blind trial assessing the effect of acupuncture in reducing musculoskeletal symptoms in breast cancer patients taking aromatase inhibitors. Breast Cancer Res Treat 138(1):167–174. doi:10.1007/s10549-013-2427-z
Chien TJ, Liu CY, Chang YF, Fang CJ, Hsu CH (2015) Acupuncture for treating aromatase inhibitor-related arthralgia in breast cancer: a systematic review and meta-analysis. J Altern Complement Med 21(5):251–260. doi:10.1089/acm.2014.0083
Hershman DL, Unger JM, Crew KD, Awad D, Dakhil SR, Gralow J, Greenlee H, Lew DL, Minasian LM, Till C, Wade JL III, Meyskens FL, Moinpour CM (2015) Randomized multicenter placebo-controlled trial of omega-3 fatty acids for the control of aromatase inhibitor-induced musculoskeletal pain: SWOG S0927. J Clin Oncol Off J Am Soc Clin Oncol 33(17):1910–1917. doi:10.1200/JCO.2014.59.5595
Briot K, Tubiana-Hulin M, Bastit L, Kloos I, Roux C (2010) Effect of a switch of aromatase inhibitors on musculoskeletal symptoms in postmenopausal women with hormone-receptor-positive breast cancer: the ATOLL (articular tolerance of letrozole) study. Breast Cancer Res Treat 120(1):127–134. doi:10.1007/s10549-009-0692-7
Desai K, Mao JJ, Su I, Demichele A, Li Q, Xie SX, Gehrman PR (2013) Prevalence and risk factors for insomnia among breast cancer patients on aromatase inhibitors. Support Care Cancer 21(1):43–51. doi:10.1007/s00520-012-1490-z
Lopez C, Charles C, Rouby P, Boinon D, Laurent S, Rey A, Spielmann M, Dauchy S (2015) Relations between arthralgia and fear of recurrence: results of a cross-sectional study of breast cancer patients treated with adjuvant aromatase inhibitors therapy. Support Care Cancer 23(12):3581–3588. doi:10.1007/s00520-015-2722-9
Peng N, Zhang Y, Ma C, Yu MW, Yang GW, Fu Q, Xu WR, Wang XM (2014) Effects of the traditional Chinese medicine Yi Shen Jian Gu granules on aromatase inhibitor-associated musculoskeletal symptoms: a study protocol for a multicenter, randomized, controlled clinical trial. Trials 15:171. doi:10.1186/1745-6215-15-171
Powe CE, Evans MK, Wenger J, Zonderman AB, Berg AH, Nalls M, Tamez H, Zhang D, Bhan I, Karumanchi SA, Powe NR, Thadhani R (2013) Vitamin D-binding protein and vitamin D status of Black Americans and White Americans. N Engl J Med 369(21):1991–2000. doi:10.1056/NEJMoa1306357
Bischoff-Ferrari HA, Willett WC, Orav EJ, Lips P, Meunier PJ, Lyons RA, Flicker L, Wark J, Jackson RD, Cauley JA, Meyer HE, Pfeifer M, Sanders KM, Stahelin HB, Theiler R, Dawson-Hughes B (2012) A pooled analysis of vitamin D dose requirements for fracture prevention. N Engl J Med 367(1):40–49. doi:10.1056/NEJMoa1109617
Cescon DW, Ganz PA, Beddows S, Ennis M, Mills BK, Goodwin PJ (2012) Feasibility of a randomized controlled trial of vitamin D vs. placebo in women with recently diagnosed breast cancer. Breast Cancer Res Treat 134(2):759–767. doi:10.1007/s10549-012-2120-7
Neuhouser ML, Sorensen B, Hollis BW, Ambs A, Ulrich CM, McTiernan A, Bernstein L, Wayne S, Gilliland F, Baumgartner K, Baumgartner R, Ballard-Barbash R (2008) Vitamin D insufficiency in a multiethnic cohort of breast cancer survivors. Am J Clin Nutr 88(1):133–139
Crew KD, Shane E, Cremers S, McMahon DJ, Irani D, Hershman DL (2009) High prevalence of vitamin D deficiency despite supplementation in premenopausal women with breast cancer undergoing adjuvant chemotherapy. J Clin Oncol Off J Am Soc Clin Oncol 27(13):2151–2156. doi:10.1200/JCO.2008.19.6162
Durup D, Jorgensen HL, Christensen J, Tjonneland A, Olsen A, Halkjaer J, Lind B, Heegaard AM, Schwarz P (2015) A reverse J-shaped association between serum 25-hydroxyvitamin D and cardiovascular disease mortality: the CopD study. J Clin Endocrinol Metab 100(6):2339–2346. doi:10.1210/jc.2014-4551
Acknowledgments
Many thanks to the study participants who made this trial possible. We also wish to thank Karen Zwicky, Lori Strayer, Jeremiah Menk, Marilyn Magadan, Elsie Anderson, Amber Egan, Niki Hoese, MaryJo Nissen, Karen Swenson, Michaela Tsai, Frank Stanczyk and Stan Patel, Roxanne Grayes, and the clinical laboratory staff at Methodist Hospital.
Funding
Research relating to this analysis was co-funded by Grants from the National Cancer Institute and the National Institutes of Health Office of Dietary Supplements (R21 CA149934) and the Park Nicollet Institute and Park Nicollet Foundation. This work was supported in part by NIHP30CA77598, using the following University of Minnesota Masonic Cancer Center Resource: Clinical Pharmacology and the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH UL1TR000114).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
No conflicts of interest reported by any of the authors.
Human rights and informed consent
All procedures performed in studies involving human participants were in accordance with the Ethical Standards of the Institutional and/or National Research Committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants for whom identifying information is included in this article.
Additional information
The study was registered in Clinical Trials.gov (NCT01509079) and conducted under IND #114046.
Trial Registration clinicaltrials.gov, identifier: NCT01509079.
Rights and permissions
About this article
Cite this article
Shapiro, A.C., Adlis, S.A., Robien, K. et al. Randomized, blinded trial of vitamin D3 for treating aromatase inhibitor-associated musculoskeletal symptoms (AIMSS). Breast Cancer Res Treat 155, 501–512 (2016). https://doi.org/10.1007/s10549-016-3710-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-016-3710-6