Abstract
Introduction
Breast cancer metastasis is the main cause of cancer-related death in women worldwide. Current therapies have remarkably improved the prognosis of breast cancer patients but still fail to manage metastatic breast cancer. Here, the present study was set to explore the role of microRNA (miR)-660 from tumor-associated macrophages (TAMs) in breast cancer, particularly in metastasis.
Materials and methods
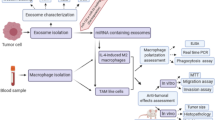
We collected breast cancer tissues and isolated their polarized macrophages as well as extracellular vesicles (EVs), in which we measured the expression of miR-660, Kelch-like Protein 21 (KLHL21), and nuclear factor-κB (NF-κB) p65. Breast cancer cells were transfected with miR-660 mimic, miR-660 inhibitor, and sh-KLHL21 and then the cells were co-cultured with EVs or TAMs followed by detection of invasion and migration. Finally, mouse model of breast cancer was established to detect the effect of miR-660 or KLHL21 on metastasis by measuring the lymph node metastasis (LNM) foci in femur and lung.
Results
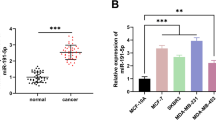
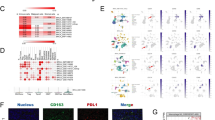
KLHL21 was poorly expressed, whereas miR-660 was highly expressed in breast cancer tissues and cells. Of note, low KLHL21 expression or high miR-660 expression was related to poor overall survival. EVs-contained miR-660 was identified to bind to KLHL21, reducing the binding between KLHL21 and inhibitor kappa B kinase β (IKKβ) to activate the NF-κB p65 signaling pathway. Interestingly, EV-loaded miR-660 from TAMs could be internalized by breast cancer cells. Moreover, silencing of KLHL21 increased the number of lung LNM foci in vivo, while EVs-contained miR-660 promoted cancerous cell invasion and migration.
Discussion
Taken altogether, our work shows that TAMs-EVs-shuttled miR-660 promotes breast cancer progression through KLHL21-mediated IKKβ/NF-κB p65 axis.









Similar content being viewed by others
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- miR:
-
microRNA
- TAMs:
-
Tumor-associated macrophages
- EVs:
-
Extracellular vesicles
- KLHL21:
-
Kelch-like protein 21
- IKKβ:
-
Inhibitor kappa B kinase β
- miRNAs:
-
microRNAs
- HE:
-
Hematoxylin–eosin
- RT-qPCR:
-
Reverse transcription quantitative polymerase chain reaction
- GAPDH:
-
Glyceraldehyde-phosphate dehydrogenase
- FBS:
-
Fetal bovine serum
- shKLHL21:
-
shRNA-Kelch-likeProtein21
- NCs:
-
Negative controls
- UTR:
-
Untranslated region
- WT:
-
Wild type
- MUT:
-
Mutant
- IHC:
-
Immunohistochemistry
- RNA-FISH:
-
RNA-fluorescence in situ hybridization
- Co-IP:
-
Co-immunoprecipitation
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6):394–424. https://doi.org/10.3322/caac.21492
Ponde NF, Zardavas D, Piccart M (2019) Progress in adjuvant systemic therapy for breast cancer. Nat Rev Clin Oncol 16(1):27–44. https://doi.org/10.1038/s41571-018-0089-9
Harbeck N, Gnant M (2017) Breast cancer. Lancet 389(10074):1134–1150. https://doi.org/10.1016/S0140-6736(16)31891-8
Penault-Llorca F, Radosevic-Robin N (2016) Biomarkers of residual disease after neoadjuvant therapy for breast cancer. Nat Rev Clin Oncol 13(8):487–503. https://doi.org/10.1038/nrclinonc.2016.1
Lapenna A, De Palma M, Lewis CE (2018) Perivascular macrophages in health and disease. Nat Rev Immunol 18(11):689–702. https://doi.org/10.1038/s41577-018-0056-9
Komohara Y, Fujiwara Y, Ohnishi K, Takeya M (2016) Tumor-associated macrophages: potential therapeutic targets for anti-cancer therapy. Adv Drug Deliv Rev 99(Pt B):180–185. https://doi.org/10.1016/j.addr.2015.11.009
Ngambenjawong C, Gustafson HH, Pun SH (2017) Progress in tumor-associated macrophage (TAM)-targeted therapeutics. Adv Drug Deliv Rev 114:206–221. https://doi.org/10.1016/j.addr.2017.04.010
Cassetta L, Fragkogianni S, Sims AH, Swierczak A, Forrester LM, Zhang H, Soong DYH, Cotechini T, Anur P, Lin EY, Fidanza A, Lopez-Yrigoyen M, Millar MR, Urman A, Ai Z, Spellman PT, Hwang ES, Dixon JM, Wiechmann L, Coussens LM, Smith HO, Pollard JW (2019) Human tumor-associated macrophage and monocyte transcriptional landscapes reveal cancer-specific reprogramming, biomarkers, and therapeutic targets. Cancer Cell 35(4):588–602. https://doi.org/10.1016/j.ccell.2019.02.009
Qiu SQ, Waaijer SJH, Zwager MC, de Vries EGE, van der Vegt B, Schroder CP (2018) Tumor-associated macrophages in breast cancer: innocent bystander or important player? Cancer Treat Rev 70:178–189. https://doi.org/10.1016/j.ctrv.2018.08.010
Chen C, Liu JM, Luo YP (2020) MicroRNAs in tumor immunity: functional regulation in tumor-associated macrophages. J Zhejiang Univ Sci B 21(1):12–28. https://doi.org/10.1631/jzus.B1900452
Bleckmann A, Leha A, Artmann S, Menck K, Salinas-Riester G, Binder C, Pukrop T, Beissbarth T, Klemm F (2015) Integrated miRNA and mRNA profiling of tumor-educated macrophages identifies prognostic subgroups in estrogen receptor-positive breast cancer. Mol Oncol 9(1):155–166. https://doi.org/10.1016/j.molonc.2014.07.023
Cianciaruso C, Beltraminelli T, Duval F, Nassiri S, Hamelin R, Mozes A, Gallart-Ayala H, Ceada Torres G, Torchia B, Ries CH, Ivanisevic J, De Palma M (2019) Molecular profiling and functional analysis of macrophage-derived tumor extracellular vesicles. Cell Rep 27(10):3062–3080. https://doi.org/10.1016/j.celrep.2019.05.008
Xu R, Rai A, Chen M, Suwakulsiri W, Greening DW, Simpson RJ (2018) Extracellular vesicles in cancer—implications for future improvements in cancer care. Nat Rev Clin Oncol 15(10):617–638. https://doi.org/10.1038/s41571-018-0036-9
Xie F, Zhou X, Fang M, Li H, Su P, Tu Y, Zhang L, Zhou F (2019) Extracellular vesicles in cancer immune microenvironment and cancer immunotherapy. Adv Sci (Weinh) 6(24):1901779. https://doi.org/10.1002/advs.201901779
Mori MA, Ludwig RG, Garcia-Martin R, Brandao BB, Kahn CR (2019) Extracellular miRNAs: from biomarkers to mediators of physiology and disease. Cell Metab 30(4):656–673. https://doi.org/10.1016/j.cmet.2019.07.011
Qi Y, Zha W, Zhang W (2019) Exosomal miR-660-5p promotes tumor growth and metastasis in non-small cell lung cancer. J BUON 24(2):599–607
Zhou H, Tang G, Zhao M, Xie L, Xie Y, Zhang Z, He X (2020) circFBXL5 promotes breast cancer progression by sponging miR-660. J Cell Mol Med 24(1):356–361. https://doi.org/10.1111/jcmm.14737
Krishnan P, Ghosh S, Wang B, Li D, Narasimhan A, Berendt R, Graham K, Mackey JR, Kovalchuk O, Damaraju S (2015) Next generation sequencing profiling identifies miR-574-3p and miR-660-5p as potential novel prognostic markers for breast cancer. BMC Genomics 16:735. https://doi.org/10.1186/s12864-015-1899-0
National Research Counci (2011) Guide for the care and use of laboratory animals, 8th edn. The National Academies Press, Washington, DC. https://doi.org/10.17226/12910
Sarrion-Perdigones A, Chang L, Gonzalez Y, Gallego-Flores T, Young DW, Venken KJT (2019) Examining multiple cellular pathways at once using multiplex hextuple luciferase assaying. Nat Commun 10(1):5710. https://doi.org/10.1038/s41467-019-13651-y
Shen Y, Ye YF, Ruan LW, Bao L, Wu MW, Zhou Y (2017) Inhibition of miR-660-5p expression suppresses tumor development and metastasis in human breast cancer. Genet Mol Res. https://doi.org/10.4238/gmr16019479
Chen J, Song W, Du Y, Li Z, Xuan Z, Zhao L, Chen J, Zhao Y, Tuo B, Zheng S, Song P (2018) Inhibition of KLHL21 prevents cholangiocarcinoma progression through regulating cell proliferation and motility, arresting cell cycle and reducing Erk activation. Biochem Biophys Res Commun 499(3):433–440. https://doi.org/10.1016/j.bbrc.2018.03.152
Mei ZZ, Chen XY, Hu SW, Wang N, Ou XL, Wang J, Luo HH, Liu J, Jiang Y (2016) Kelch-like protein 21 (KLHL21) targets IkappaB kinase-beta to regulate nuclear factor kappa-light chain enhancer of activated B cells (NF-kappaB) signaling negatively. J Biol Chem 291(35):18176–18189. https://doi.org/10.1074/jbc.M116.715854
Yang M, Chen J, Su F, Yu B, Su F, Lin L, Liu Y, Huang JD, Song E (2011) Microvesicles secreted by macrophages shuttle invasion-potentiating microRNAs into breast cancer cells. Mol Cancer 10:117. https://doi.org/10.1186/1476-4598-10-117
Frankenberger C, Rabe D, Bainer R, Sankarasharma D, Chada K, Krausz T, Gilad Y, Becker L, Rosner MR (2015) Metastasis suppressors regulate the tumor microenvironment by blocking recruitment of prometastatic tumor-associated macrophages. Cancer Res 75(19):4063–4073. https://doi.org/10.1158/0008-5472.CAN-14-3394
Nicoloso MS, Spizzo R, Shimizu M, Rossi S, Calin GA (2009) MicroRNAs–the micro steering wheel of tumour metastases. Nat Rev Cancer 9(4):293–302. https://doi.org/10.1038/nrc2619
Nashtahosseini Z, Aghamaali MR, Sadeghi F, Heydari N, Parsian H (2021) Circulating status of microRNAs 660–5p and 210–3p in breast cancer patients. J Gene Med 23(4):e3320. https://doi.org/10.1002/jgm.3320
Peng B, Li C, He L, Tian M, Li X (2020) miR-660-5p promotes breast cancer progression through down-regulating TET2 and activating PI3K/AKT/mTOR signaling. Braz J Med Biol Res 53(12):e9740. https://doi.org/10.1590/1414-431X20209740
Riggi N, Aguet M, Stamenkovic I (2018) Cancer metastasis: a reappraisal of its underlying mechanisms and their relevance to treatment. Annu Rev Pathol 13:117–140. https://doi.org/10.1146/annurev-pathol-020117-044127
Dongre A, Weinberg RA (2019) New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat Rev Mol Cell Biol 20(2):69–84. https://doi.org/10.1038/s41580-018-0080-4
Pastushenko I, Blanpain C (2019) EMT transition states during tumor progression and metastasis. Trends Cell Biol 29(3):212–226. https://doi.org/10.1016/j.tcb.2018.12.001
Ashaie MA, Chowdhury EH (2016) Cadherins: the superfamily critically involved in breast cancer. Curr Pharm Des 22(5):616–638. https://doi.org/10.2174/138161282205160127095338
Padmanaban V, Krol I, Suhail Y, Szczerba BM, Aceto N, Bader JS, Ewald AJ (2019) E-cadherin is required for metastasis in multiple models of breast cancer. Nature 573(7774):439–444. https://doi.org/10.1038/s41586-019-1526-3
Hsu CC, Huang SF, Wang JS, Chu WK, Nien JE, Chen WS, Chow SE (2016) Interplay of N-Cadherin and matrix metalloproteinase 9 enhances human nasopharyngeal carcinoma cell invasion. BMC Cancer 16(1):800. https://doi.org/10.1186/s12885-016-2846-4
Radisky DC, Levy DD, Littlepage LE, Liu H, Nelson CM, Fata JE, Leake D, Godden EL, Albertson DG, Nieto MA, Werb Z, Bissell MJ (2005) Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature 436(7047):123–127. https://doi.org/10.1038/nature03688
Padala C, Tupurani MA, Puranam K, Gantala S, Shyamala N, Kondapalli MS, Gundapaneni KK, Mudigonda S, Galimudi RK, Kupsal K, Nanchari SR, Chavan U, Chinta SK, Mukta S, Satti V, Hanumanth SR (2017) Synergistic effect of collagenase-1 (MMP1), stromelysin-1 (MMP3) and gelatinase-B (MMP9) gene polymorphisms in breast cancer. PLoS ONE 12(9):e0184448. https://doi.org/10.1371/journal.pone.0184448
Linde N, Casanova-Acebes M, Sosa MS, Mortha A, Rahman A, Farias E, Harper K, Tardio E, Reyes Torres I, Jones J, Condeelis J, Merad M, Aguirre-Ghiso JA (2018) Macrophages orchestrate breast cancer early dissemination and metastasis. Nat Commun 9(1):21. https://doi.org/10.1038/s41467-017-02481-5
Su S, Liu Q, Chen J, Chen J, Chen F, He C, Huang D, Wu W, Lin L, Huang W, Zhang J, Cui X, Zheng F, Li H, Yao H, Su F, Song E (2014) A positive feedback loop between mesenchymal-like cancer cells and macrophages is essential to breast cancer metastasis. Cancer Cell 25(5):605–620. https://doi.org/10.1016/j.ccr.2014.03.021
Lin YW, Lee LM, Lee WJ, Chu CY, Tan P, Yang YC, Chen WY, Yang SF, Hsiao M, Chien MH (2016) Melatonin inhibits MMP-9 transactivation and renal cell carcinoma metastasis by suppressing Akt-MAPKs pathway and NF-kappaB DNA-binding activity. J Pineal Res 60(3):277–290. https://doi.org/10.1111/jpi.12308
Fusella F, Secli L, Busso E, Krepelova A, Moiso E, Rocca S, Conti L, Annaratone L, Rubinetto C, Mello-Grand M, Singh V, Chiorino G, Silengo L, Altruda F, Turco E, Morotti A, Oliviero S, Castellano I, Cavallo F, Provero P, Tarone G, Brancaccio M (2017) The IKK/NF-kappaB signaling pathway requires Morgana to drive breast cancer metastasis. Nat Commun 8(1):1636. https://doi.org/10.1038/s41467-017-01829-1
Dembinski HE, Wismer K, Vargas JD, Suryawanshi GW, Kern N, Kroon G, Dyson HJ, Hoffmann A, Komives EA (2017) Functional importance of stripping in NFkappaB signaling revealed by a stripping-impaired IkappaBalpha mutant. Proc Natl Acad Sci USA 114(8):1916–1921. https://doi.org/10.1073/pnas.1610192114
Marino S, Bishop RT, Logan JG, Mollat P, Idris AI (2017) Pharmacological evidence for the bone-autonomous contribution of the NFkappaB/beta-catenin axis to breast cancer related osteolysis. Cancer Lett 410:180–190. https://doi.org/10.1016/j.canlet.2017.09.034
Ell B, Mercatali L, Ibrahim T, Campbell N, Schwarzenbach H, Pantel K, Amadori D, Kang Y (2013) Tumor-induced osteoclast miRNA changes as regulators and biomarkers of osteolytic bone metastasis. Cancer Cell 24(4):542–556. https://doi.org/10.1016/j.ccr.2013.09.008
Hirschfeld M, Rucker G, Weiss D, Berner K, Ritter A, Jager M, Erbes T (2020) Urinary exosomal MicroRNAs as potential non-invasive biomarkers in breast cancer detection. Mol Diagn Ther 24(2):215–232. https://doi.org/10.1007/s40291-020-00453-y
Munoz-Rodriguez JL, Vrba L, Futscher BW, Hu C, Komenaka IK, Meza-Montenegro MM, Gutierrez-Millan LE, Daneri-Navarro A, Thompson PA, Martinez ME (2015) Differentially expressed microRNAs in postpartum breast cancer in Hispanic women. PLoS ONE 10(4):e0124340. https://doi.org/10.1371/journal.pone.0124340
Nassar FJ, Nasr R, Talhouk R (2017) MicroRNAs as biomarkers for early breast cancer diagnosis, prognosis and therapy prediction. Pharmacol Ther 172:34–49. https://doi.org/10.1016/j.pharmthera.2016.11.012
Acknowledgements
We would like to give our sincere appreciation to the reviewers for their helpful comments on this article.
Funding
This work was supported by grants from the Suzhou Health Planning Commission’s Key Clinical Diagnosis and Treatment Program (Grant No. LCZX201606), National Natural Science Foundation of China (Grant No. 81873730) and the Jiangsu Women and Children Health Key Discipline Program (Grant No. FXK201758).
Author information
Authors and Affiliations
Contributions
(I) Conception and design: CL, RL; (II) Administrative support: CL, XH; (III) Provision of study materials or patients: CL, GJ; (IV) Collection and assembly of data: RL, GZ, GJ; (V) Data analysis and interpretation: RL, XH, GZ; (VI) Manuscript writing: All authors; (VII) Final approval of manuscript: All authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
The experiment was approved by the Ethics Committee of the Second Affiliated Hospital of Soochow University and conducted following the Declaration of Helsinki. All individuals signed informed written consent documents. The experiments involving animals followed the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10549_2021_6433_MOESM4_ESM.jpg
Supplementary Fig. 2 Correlation analysis of KLHL21 and miR-660 level (A), KLHL21 and NF-κB p65 level (B) in adjacent normal tissues and breast cancer tissues. (JPG 611 kb)
10549_2021_6433_MOESM5_ESM.jpg
Supplementary Fig. 3 The original gel image of E-cadherin, N-cadherin, MMP-9, MMP-3, and β-actin in breast cancer cells treated with sh-KLHL21, miR-660 inhibitor, or controls. (JPG 1364 kb)
10549_2021_6433_MOESM6_ESM.jpg
Supplementary Fig. 4 HE staining of lung LNM foci from mice following all treatments (A) and quantification of number of metastases in vivoe.Supplementary Fig. 4 HE staining of lung LNM foci from mice following all treatments (A) and quantification of number of metastases in vivo. (JPG 2023 kb)
10549_2021_6433_MOESM7_ESM.jpg
Supplementary Fig. 5 Representative protein blots of Western blot analysis. A, Protein blots of Fig. 3C. B, Protein blots of Fig. 4B. C, Protein blots of Fig. 6B, D, Protein blots of Fig. 6D. E, Protein blots of Fig. 6G. F, Protein blots of Fig. 7A. G, Protein blots of Fig. 7B. (JPG 1314 kb)
Rights and permissions
About this article
Cite this article
Li, C., Li, R., Hu, X. et al. Tumor-promoting mechanisms of macrophage-derived extracellular vesicles-enclosed microRNA-660 in breast cancer progression. Breast Cancer Res Treat 192, 353–368 (2022). https://doi.org/10.1007/s10549-021-06433-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-021-06433-y