Abstract
Purpose
The role of zoledronic acid (ZOL), a bone-targeted bisphosphonate, in the treatment of patients with breast cancer remains an active area of study. Here, we report the long-term outcomes of a randomized placebo-controlled phase II clinical trial in which ZOL treatment was added to neoadjuvant chemotherapy in women with locally advanced breast cancer.
Methods
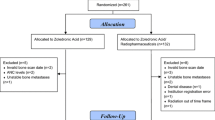
120 women with clinical stage II–III (≥ T2 and/or ≥ N1) newly diagnosed breast cancer were randomized to receive either 4 mg intravenous ZOL every 3 weeks for 1 year (17 total doses) beginning with the first dose of neoadjuvant chemotherapy, or chemotherapy alone. Clinical endpoints included time to recurrence (TTR), time to bone recurrence (TTBR), time to non-bone recurrence (TTNBR), breast cancer survival (BCS) and overall survival (OS).
Results
With a median follow-up interval of 14.4 years, there were no significant differences in any of the clinical endpoints studied between the control and ZOL groups in the overall study population. However, ER+/HER2− patients younger than age 45 who were treated with ZOL had significantly worse TTR and TTNBR with a trend towards worse TTBR, BCS and OS (TTR: P = 0.024, HR 6.05 [1.26–29.1]; TTNBR: P = 0.026, HR 6.94 [1.26–38.1]; TTBR: P = 0.054, HR 6.01 [0.97–37.1]; BCS: P = 0.138, HR 4.43 [0.62–31.7]; OS: P = 0.138, HR 4.43 [0.62–31.7]). These differences were not seen in older ER+/HER2− patients or triple-negative patients of any age.
Conclusion
Addition of ZOL to neoadjuvant therapy did not significantly affect clinical outcomes in the overall study population but was associated with increased extra-skeletal recurrence and a trend towards worse survival in ER+/HER2− patients younger than age 45. These findings suggest caution when using zoledronic acid in young, premenopausal women with locally advanced breast cancer and warrant further investigation.
Clinical Trial Registration Number NCT00242203, Date of Registration: 10/17/2005





Similar content being viewed by others
Data availability
Deidentified datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Siegel RL, Miller KD, Jemal A (2019) Cancer statistics, 2019. CA A Cancer J Clin 69:7–34. https://doi.org/10.3322/caac.21551
Dent R, Hanna WM, Trudeau M, Rawlinson E, Sun P, Narod SA (2009) Pattern of metastatic spread in triple-negative breast cancer. Breast Cancer Res Treat 115:423–428. https://doi.org/10.1007/s10549-008-0086-2
Reddy SM, Barcenas CH, Sinha AK, Hsu L, Moulder SL, Tripathy D, Hortobagyi GN, Valero V (2018) Long-term survival outcomes of triple-receptor negative breast cancer survivors who are disease free at 5 years and relationship with low hormone receptor positivity. Br J Cancer 118:17–23. https://doi.org/10.1038/bjc.2017.379
Pan H, Gray R, Braybrooke J, Davies C, Taylor C, McGale P, Peto R, Pritchard KI, Bergh J, Dowsett M, Hayes DF (2017) 20-year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. N Engl J Med 377:1836–1846. https://doi.org/10.1056/NEJMoa1701830
Braun S, Janni W, Schlimok G, Gebauer G, Oruzio D, Kundt G, Wong GYC, Pantel K (2005) A pooled analysis of bone marrow micrometastasis in breast cancer. New Engl J Med 353:793
Price TT, Burness ML, Sivan A, Warner MJ, Cheng R, Lee CH, Olivere L, Comatas K, Magnani J, Lyerly HK, Cheng Q, McCall CM, Sipkins DA (2016) Dormant breast cancer micrometastases reside in specific bone marrow niches that regulate their transit to and from bone. Sci Transl Med 8:340ra73. https://doi.org/10.1126/scitranslmed.aad4059
Weilbaecher KN, Guise TA, McCauley LK (2011) Cancer to bone: a fatal attraction. Nat Rev Cancer 11:411–425. https://doi.org/10.1038/nrc3055
Aft R, Naughton M, Trinkaus K, Watson M, Ylagan L, Chavez-MacGregor M, Zhai J, Kuo S, Shannon W, Diemer K, Herrmann V, Dietz J, Ali A, Ellis M, Weiss P, Eberlein T, Ma C, Fracasso PM, Zoberi I, Taylor M, Gillanders W, Pluard T, Mortimer J, Weilbaecher K (2010) Effect of zoledronic acid on disseminated tumour cells in women with locally advanced breast cancer: an open label, randomised, phase 2 trial. Lancet Oncol 11:421–428. https://doi.org/10.1016/S1470-2045(10)70054-1
Banys M, Solomayer E-F, Gebauer G, Janni W, Krawczyk N, Lueck H-J, Becker S, Huober J, Kraemer B, Wackwitz B, Hirnle P, Wallwiener D, Fehm T (2013) Influence of zoledronic acid on disseminated tumor cells in bone marrow and survival: results of a prospective clinical trial. BMC Cancer 13:480. https://doi.org/10.1186/1471-2407-13-480
Rack B, Jückstock J, Genss E-M, Schoberth A, Schindlbeck C, Strobl B, Heinrigs M, Rammel G, Zwingers T, Sommer H, Friese K, Janni W (2010) Effect of zoledronate on persisting isolated tumour cells in patients with early breast cancer. Anticancer Res 30:1807–1813
Coleman R, Cameron D, Dodwell D, Bell R, Wilson C, Rathbone E, Keane M, Gil M, Burkinshaw R, Grieve R, Barrett-Lee P, Ritchie D, Liversedge V, Hinsley S, Marshall H (2014) Adjuvant zoledronic acid in patients with early breast cancer: final efficacy analysis of the AZURE (BIG 01/04) randomised open-label phase 3 trial. Lancet Oncol 15:997–1006. https://doi.org/10.1016/S1470-2045(14)70302-X
Coleman R, de Boer R, Eidtmann H, Llombart A, Davidson N, Neven P, von Minckwitz G, Sleeboom HP, Forbes J, Barrios C, Frassoldati A, Campbell I, Paija O, Martin N, Modi A, Bundred N (2013) Zoledronic acid (zoledronate) for postmenopausal women with early breast cancer receiving adjuvant letrozole (ZO-FAST study): final 60-month results. Ann Oncol 24:398–405. https://doi.org/10.1093/annonc/mds277
Gnant M, Mlineritsch B, Stoeger H, Luschin-Ebengreuth G, Heck D, Menzel C, Jakesz R, Seifert M, Hubalek M, Pristauz G, Bauernhofer T, Eidtmann H, Eiermann W, Steger G, Kwasny W, Dubsky P, Hochreiner G, Forsthuber E-P, Fesl C, Greil R (2011) Adjuvant endocrine therapy plus zoledronic acid in premenopausal women with early-stage breast cancer: 62-month follow-up from the ABCSG-12 randomised trial. Lancet Oncol 12:631–641. https://doi.org/10.1016/S1470-2045(11)70122-X
Coleman RE, Collinson M, Gregory W, Marshall H, Bell R, Dodwell D, Keane M, Gil M, Barrett-Lee P, Ritchie D, Bowman A, Liversedge V, De Boer RH, Passos-Coelho JL, O’Reilly S, Bertelli G, Joffe J, Brown JE, Wilson C, Tercero JC, Jean-Mairet J, Gomis R, Cameron D (2018) Benefits and risks of adjuvant treatment with zoledronic acid in stage II/III breast cancer. 10 years follow-up of the AZURE randomized clinical trial (BIG 01/04). J Bone Oncol 13:123–135. https://doi.org/10.1016/j.jbo.2018.09.008
Aft RL, Naughton M, Trinkaus K, Weilbaecher K (2012) Effect of (Neo)adjuvant zoledronic acid on disease-free and overall survival in clinical stage II/III breast cancer. Br J Cancer 107:7–11. https://doi.org/10.1038/bjc.2012.210
Hillner BE, Ingle JN, Chlebowski RT, Gralow J, Yee GC, Janjan NA, Cauley JA, Blumenstein BA, Albain KS, Lipton A, Brown S (2003) American society of clinical oncology 2003 update on the role of bisphosphonates and bone health issues in women with breast cancer. JCO 21:4042–4057. https://doi.org/10.1200/JCO.2003.08.017
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) (2005) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. The Lancet 365:1687–1717. https://doi.org/10.1016/S0140-6736(05)66544-0
de Groot S, Pijl H, Charehbili A, van de Ven S, Smit VTHBM, Meershoek-Klein Kranenbarg E, Heijns JB, van Warmerdam LJC, Kessels LW, Dercksen MW, Pepels MJAE, van Laarhoven HWM, Vriens BEPJ, Putter H, Fiocco M, Liefers G-J, van der Hoeven JJM, Nortier JWR, Kroep JR, on behalf of the Dutch Breast Cancer Research Group (2019) Addition of zoledronic acid to neoadjuvant chemotherapy is not beneficial in patients with HER2-negative stage II/III breast cancer: 5-year survival analysis of the NEOZOTAC trial (BOOG 2010–01). Breast Cancer Res 21:97. https://doi.org/10.1186/s13058-019-1180-6
Saarto T, Vehmanen L, Virkkunen P, Blomqvist C (2004) Ten-year follow-up of a randomized controlled trial of adjuvant clodronate treatment in node-positive breast cancer patients. Acta Oncol 43:650–656. https://doi.org/10.1080/02841860410032885
Paterson AH, Anderson SJ, Lembersky BC, Fehrenbacher L, Falkson CI, King KM, Weir LM, Brufsky AM, Dakhil S, Lad T, Baez-Diaz L, Gralow JR, Robidoux A, Perez EA, Zheng P, Geyer CE, Swain SM, Costantino JP, Mamounas EP, Wolmark N (2012) Oral clodronate for adjuvant treatment of operable breast cancer (National Surgical Adjuvant Breast and Bowel Project protocol B-34): a multicentre, placebo-controlled, randomised trial. Lancet Oncol 13:734–742. https://doi.org/10.1016/S1470-2045(12)70226-7
Charehbili A, van de Ven S, Smit V, Kranenbarg EM-K, Hamdy N, Putter H, Heijns J, van Warmerdam L, Kessels L, Dercksen M, Pepels M, Maartense E, van Laarhoven H, Vriens B, Wasser M, van Leeuwen-Stok A, Liefers G, van de Velde C, Nortier J, Kroep J (2014) Addition of zoledronic acid to neoadjuvant chemotherapy does not enhance tumor response in patients with HER2-negative stage II/III breast cancer: the NEOZOTAC trial (BOOG 2010–01). Ann Oncol 25:998–1004. https://doi.org/10.1093/annonc/mdu102
Ottewell PD, Wang N, Brown HK, Reeves KJ, Fowles CA, Croucher PI, Eaton CL, Holen I (2014) Zoledronic acid has differential antitumor activity in the pre- and postmenopausal bone microenvironment in vivo. Clin Cancer Res 20:2922–2932. https://doi.org/10.1158/1078-0432.CCR-13-1246
Steinman RA, Brufsky AM, Oesterreich S (2012) Zoledronic acid effectiveness against breast cancer metastases—a role for estrogen in the microenvironment? Breast Cancer Res 14:213. https://doi.org/10.1186/bcr3223
Summers MA, McDonald MM, Croucher PI (2020) Cancer cell dormancy in metastasis. Cold Spring Harb Perspect Med 10:a037556. https://doi.org/10.1101/cshperspect.a037556
Coleman R, Hall A, Albanell J, Hanby A, Bell R, Cameron D, Dodwell D, Marshall H, Jean-Mairet J, Tercero J-C, Rojo F, Gregory W, Gomis RR (2017) Effect of MAF amplification on treatment outcomes with adjuvant zoledronic acid in early breast cancer: a secondary analysis of the international, open-label, randomised, controlled, phase 3 AZURE (BIG 01/04) trial. Lancet Oncol 18:1543–1552. https://doi.org/10.1016/S1470-2045(17)30603-4
Silverman SL (2011) Defining Zoledronate’s duration of action and optimal dosing interval for an effective therapy. Curr Osteoporos Rep 9:4–5. https://doi.org/10.1007/s11914-010-0044-x
Funding
This work was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number TL1TR002344. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
AJ, KW and RA contributed to the study conception and design. Material preparation, data collection and analysis were performed by AJ and SP. The first draft of the manuscript was written by AJ and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The Institutional Review Board of Washington University approved the study. The Siteman Cancer Center’s quality assurance and safety monitoring committee oversaw patient safety and all aspects of the study were conducted in accordance with the Declaration of Helsinki.
Consent to participate
Written informed consent was obtained from all individual participants included in the study.
Consent for publication
Written informed consent was obtained from all individual participants included in the study. No identifying information about participants is included in this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jallouk, A.P., Paravastu, S., Weilbaecher, K. et al. Long-term outcome of (neo)adjuvant zoledronic acid therapy in locally advanced breast cancer. Breast Cancer Res Treat 187, 135–144 (2021). https://doi.org/10.1007/s10549-021-06100-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-021-06100-2