Abstract
Purpose
Neoadjuvant endocrine therapy (NET) has been shown to be effective in ER-positive/HER2-negative breast cancer in clinical trials. However, adoption in clinical practice is still limited. Real-world data may provide useful insights into effectiveness, toxicities and quality of care, potentially rendering clinical trial results to the real-world setting. Our purpose was to report real-world data of a cohort of postmenopausal patients submitted to NET.
Methods
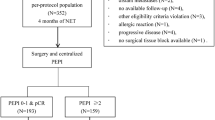
This prospective cohort study evaluated 146 postmenopausal female patients with ER-positive/HER2-negative breast cancer treated with NET at three tertiary hospitals between 2016 and 2018. Clinicopathological information were collected prospectively. Preoperative Endocrine Prognostic Index (PEPI) score was calculated for tumors submitted to at least 16 weeks of NET.
Results
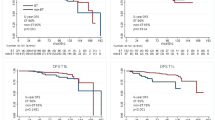
Median age was 67 years old, and 87.8% had stage I-II disease. Most tumors had histological grade II (76.1%). Median pretreatment Ki67 expression was 10%. Aromatase inhibitor was used in 99.5% of patients, and median treatment duration was 21.0 weeks. No tumor progressed during NET. Breast-conserving surgery was performed in the majority of patients (63.0%), as well as sentinel lymph-node biopsy (76.7%). Pathological complete response rate was 1.0%. 43 patients (29.5%) had PEPI score 0, and 26% had PEPI scores 4–5. Posttreatment Ki67 median expression was 3.0%, and only five tumors (3.4%) showed marked increase in Ki67 expression during treatment. Seven patients (4.8%) had HER2-positive residual disease, and were treated with adjuvant chemotherapy plus trastuzumab.
Conclusions
Our real-world data shows that NET is effective and safe in postmenopausal patients with ER-positive/HER2-negative breast cancer. Postmenopausal status and low-risk luminal tumor features (luminal A-like) should be used as selection criteria to ensure the best results with NET.


Similar content being viewed by others
Data availability
All the authors had full access to all the data in the study and vouch for the decision to submit the manuscript for publication.
References
Chia YH, Ellis MJ, Ma CX (2010) Neoadjuvant endocrine therapy in primary breast cancer: indications and use as a research tool. Brit J Cancer 103(6):759–764
Reinert T, Ramalho S, Gonçalves R, Barrios CH, Graudenz MS, Bines J (2016) Multidisciplinary approach to neoadjuvant endocrine therapy in breast cancer: a comprehensive review. Rev Bras Ginecol Obstet Rev Bras Soc Ginecol Obstet 38(12):615–622
Eiermann W, Paepke S, Appfelstaedt J, Llombart-Cussac A, Eremin J, Vinholes J et al (2001) Preoperative treatment of postmenopausal breast cancer patients with letrozole: a randomized double-blind multicenter study. Ann Oncol 12(11):1527–1532
Smith IE, Dowsett M, Ebbs SR, Dixon JM, Skene A, Blohmer J-U et al (2005) Neoadjuvant treatment of postmenopausal breast cancer with anastrozole, tamoxifen, or both in combination: the immediate preoperative anastrozole, tamoxifen, or combined with tamoxifen (IMPACT) multicenter double-blind randomized trial. J Clin Oncol 23(22):5108–5116
Cataliotti L, Buzdar AU, Noguchi S, Bines J, Takatsuka Y, Petrakova K et al (2006) Comparison of anastrozole versus tamoxifen as preoperative therapy in postmenopausal women with hormone receptor-positive breast cancer: the pre-operative “Arimidex” compared to Tamoxifen (PROACT) trial. Cancer 106(10):2095–2103
Sheri A, Smith IE, Johnston SR, A’Hern R, Nerurkar A, Jones RL et al (2015) Residual proliferative cancer burden to predict long-term outcome following neoadjuvant chemotherapy. Ann Oncol Off J Eur Soc Med Oncol 26(1):75–80
Symmans WF, Peintinger F, Hatzis C, Rajan R, Kuerer H, Valero V et al (2007) Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol 25(28):4414–4422
Spring LM, Fell G, Arfe A, Sharma C, Greenup R, Reynolds KL et al (2020) Pathologic complete response after neoadjuvant chemotherapy and impact on breast cancer recurrence and survival: a comprehensive meta-analysis. Clin Cancer Res 26(12):2838–2848
Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N et al (2014) Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 384(9938):164–172
Masuda N, Lee S-J, Ohtani S, Im Y-H, Lee E-S, Yokota I et al (2017) Adjuvant capecitabine for breast cancer after preoperative chemotherapy. New Engl J Med 376(22):2147–2159
von Minckwitz G, Huang C-S, Mano MS, Loibl S, Mamounas EP, Untch M et al (2019) Trastuzumab emtansine for residual invasive HER2-positive breast cancer. New Engl J Med 380(7):617–628
Ellis MJ, Suman VJ, Hoog J, Lin L, Snider J, Prat A et al (2011) Randomized phase II neoadjuvant comparison between letrozole, anastrozole, and exemestane for postmenopausal women with estrogen receptor-rich stage 2 to 3 breast cancer: clinical and biomarker outcomes and predictive value of the baseline PAM50-based intrinsic subtype–ACOSOG Z1031. J Clin Oncol Off J Am Soc Clin Oncol 29(17):2342–2349
Gonzalez-Angulo AM, Iwamoto T, Liu S, Chen H, Do K-A, Hortobagyi GN et al (2012) Gene expression, molecular class changes, and pathway analysis after neoadjuvant systemic therapy for breast cancer. Clin Cancer Res Off J Am Assoc Cancer Res 18(4):1109–1119
Ellis MJ, Tao Y, Luo J, A’Hern R, Evans DB, Bhatnagar AS et al (2008) Outcome prediction for estrogen receptor-positive breast cancer based on postneoadjuvant endocrine therapy tumor characteristics. J Natl Cancer I 100(19):1380–1388
Eichler H-G, Abadie E, Breckenridge A, Flamion B, Gustafsson LL, Leufkens H et al (2011) Bridging the efficacy-effectiveness gap: a regulator’s perspective on addressing variability of drug response. Nat Rev Drug Discov 10(7):495–506
Booth CM, Karim S, Mackillop WJ (2019) Real-world data: towards achieving the achievable in cancer care. Nat Rev Clin Oncol 16(5):312–325
Katkade VB, Sanders KN, Zou KH (2018) Real world data: an opportunity to supplement existing evidence for the use of long-established medicines in health care decision making. J Multidiscip Healthc 11:295–304
Sherman RE, Anderson SA, Pan GJD, Gray GW, Gross T, Hunter NL et al (2016) Real-world evidence—what is it and what can it tell us? New Engl J Med 375(23):2293–2297
Hammond MEH, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S et al (2010) American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol Off J Am Soc Clin Oncol 28(16):2784–2795
Allison KH, Hammond MEH, Dowsett M, McKernin SE, Carey LA, Fitzgibbons PL et al (2020) Estrogen and progesterone receptor testing in breast cancer: ASCO/CAP guideline update. J Clin Oncol Off J Am Soc Clin Oncol. https://doi.org/10.1200/JCO.19.02309
Wolff AC, Hammond MEH, Hicks DG, Dowsett M, McShane LM, Allison KH et al (2013) Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice guideline update. J Clin Oncol 31(31):3997–4013
Dowsett M, Nielsen TO, A’Hern R, Bartlett J, Coombes RC, Cuzick J et al (2011) Assessment of Ki67 in breast cancer: recommendations from the International Ki67 in Breast Cancer working group. J Natl Cancer I 103(22):1656–1664
Giuliano AE, Connolly JL, Edge SB, Mittendorf EA, Rugo HS, Solin LJ et al (2017) Breast cancer-major changes in the American Joint Committee on cancer eighth edition cancer staging manual. Ca Cancer J Clin 67(4):290–303
Goncalves R, Ma C, Luo J, Suman V, Ellis MJ (2012) Use of neoadjuvant data to design adjuvant endocrine therapy trials for breast cancer. Nat Rev Clin Oncol 9(4):223–229
Dowsett M, Ebbs SR, Dixon JM, Skene A, Griffith C, Boeddinghaus I et al (2005) Biomarker changes during neoadjuvant anastrozole, tamoxifen, or the combination: influence of hormonal status and HER-2 in breast cancer—a study from the IMPACT trialists. J Clin Oncol 23(11):2477–2492
Dixon JM, Renshaw L, Macaskill EJ, Young O, Murray J, Cameron D et al (2008) Increase in response rate by prolonged treatment with neoadjuvant letrozole. Breast Cancer Res Tr 113(1):145–151
Fontein DBY, Charehbili A, Nortier JWR, Kranenbarg EM-K, Kroep JR, Putter H et al (2014) Efficacy of six month neoadjuvant endocrine therapy in postmenopausal, hormone receptor-positive breast cancer patients—a phase II trial. Eur J Cancer. 50(13):2190–2200
Krainick-Strobel UE, Lichtenegger W, Wallwiener D, Tulusan AH, Jänicke F, Bastert G et al (2008) Neoadjuvant letrozole in postmenopausal estrogen and/or progesterone receptor positive breast cancer: a phase IIb/III trial to investigate optimal duration of preoperative endocrine therapy. BMC Cancer 8(1):62
Carpenter R, Doughty JC, Cordiner C, Moss N, Gandhi A, Wilson C et al (2014) Optimum duration of neoadjuvant letrozole to permit breast conserving surgery. Breast Cancer Res Tr 144(3):569–576
Semiglazov VF, Semiglazov VV, Dashyan GA, Ziltsova EK, Ivanov VG, Bozhok AA et al (2007) Phase 2 randomized trial of primary endocrine therapy versus chemotherapy in postmenopausal patients with estrogen receptor-positive breast cancer. Cancer 110(2):244–254
Alba E, Calvo L, Albanell J, la Haba JRD, Lanza AA, Chacon JI et al (2012) Chemotherapy (CT) and hormonotherapy (HT) as neoadjuvant treatment in luminal breast cancer patients: results from the GEICAM/2006-03, a multicenter, randomized, phase-II study. Ann Oncol Off J Eur Soc Med Oncol 23(12):3069–3074
Palmieri C, Cleator S, Kilburn LS, Kim SB, Ahn S-H, Beresford M et al (2014) NEOCENT: a randomised feasibility and translational study comparing neoadjuvant endocrine therapy with chemotherapy in ER-rich postmenopausal primary breast cancer. Breast Cancer Res Tr 148(3):581–590
Ellis MJ, Suman VJ, Hoog J, Goncalves R, Sanati S, Creighton CJ et al (2017) Ki67 Proliferation index as a tool for chemotherapy decisions during and after neoadjuvant aromatase inhibitor treatment of breast cancer: results from the American College of Surgeons Oncology Group Z1031 Trial (Alliance). J Clin Oncol 35(10):1061–1069
Suman VJ, Ellis MJ, Ma CX (2015) The ALTERNATE trial: assessing a biomarker driven strategy for the treatment of post-menopausal women with ER+/Her2- invasive breast cancer. Chin Clin Oncol 4(3):34
Niikura N, Tomotaki A, Miyata H, Iwamoto T, Kawai M, Anan K et al (2015) Changes in tumor expression of HER2 and hormone receptors status after neoadjuvant chemotherapy in 21,755 patients from the Japanese breast cancer registry. Ann Oncol Off J Eur Soc Med Oncol Esmo 27(3):480–487
Dowsett M, Ellis MJ, Dixon JM, Gluz O, Robertson J, Kates R et al (2020) Evidence-based guidelines for managing patients with primary ER+ HER2- breast cancer deferred from surgery due to the COVID-19 pandemic. NPJ Breast Cancer 6(1):21
Iwata H, Masuda N, Yamamoto Y, Fujisawa T, Toyama T, Kashiwaba M et al (2018) Validation of the 21-gene test as a predictor of clinical response to neoadjuvant hormonal therapy for ER+, HER2-negative breast cancer: the TransNEOS study. Breast Cancer Res Tr 173(1):123–133
Funding
Silva LR—Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES)—Brasil Finance Code 001. The funding sources did not have any direct involvement on the collection, analysis, and interpretation of the data, nor did they were involved on the writing and revision of the manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation and data collection were performed by LRS, CAA, SR, TR, ABAS, AERS, MBPLK, VCAV. Analysis was performed by LRS, CAA, FB, SR. The first draft of the manuscript was written by LRS, CAA, FB, SR. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interests
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Leonardo Roberto da Silva and Camila Annicchino de Andrade are co-first authors.
Rights and permissions
About this article
Cite this article
da Silva, L.R., de Andrade, C.A., Brenelli, F. et al. Real-world data on neoadjuvant endocrine therapy in ER-positive/HER2-negative breast cancer. Breast Cancer Res Treat 186, 753–760 (2021). https://doi.org/10.1007/s10549-020-06076-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-020-06076-5