Abstract
Purpose
The addition of trastuzumab to adjuvant chemotherapy has improved the outcome of human epidermal growth-factor receptor 2 (HER2)-positive breast cancer. Uncertainty remains about the optimal timing of trastuzumab treatment. Therefore, we compared long-term outcome after concurrent versus sequential treatment, in a population-based setting, using data from the nationwide Netherlands Cancer Registry.
Methods
We identified 1843 women diagnosed in The Netherlands from January 1st 2005 until January 1st 2008 with primary, HER2-positive, T1-4NanyM0 breast cancer who received adjuvant chemotherapy and trastuzumab. Kaplan–Meier survival estimates and Cox regression were used to compare recurrence-free survival (RFS) and overall survival (OS) between women who received trastuzumab concurrently with versus sequentially after chemotherapy. Hazard ratios (HR) were adjusted for age, year of diagnosis, grade, pathological T-stage, number of positive lymph nodes, ER-status, PR-status, socio-economic status, radiotherapy, hormonal therapy, ovarian ablation, and type of chemotherapy.
Results
After a median follow-up of 8.2 years, RFS events had occurred in 224 out of 1235 (18.1%) concurrently treated women and 129 out of 608 (21.2%) sequentially treated women (adjusted-HR 0.91; 95% confidence interval (CI) 0.67–1.24; P = 0.580). Deaths occurred in 182/1235 (14.7%) concurrently treated women and 104/608 (17.1%) sequentially treated women (adjusted-HR 0.92; 95% CI 0.65–1.29; P = 0.635).
Conclusions
The results of this population-based study are consistent with earlier randomized trials, demonstrating a non-significant difference in outcome for concurrently treated women compared to those who were treated sequentially, suggesting both options are justified.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
The introduction of trastuzumab, a monoclonal antibody targeting the extracellular domain of the human epidermal growth-factor receptor 2 (HER2), revolutionized the treatment of women with HER2+ breast cancer, who were among those with the poorest prognosis. Several studies, conducted in the adjuvant setting, showed impressive improvements in long-term outcome with the addition of trastuzumab to adjuvant chemotherapy [1,2,3,4,5,6,7]. Trastuzumab has, therefore, been incorporated in both national and international guidelines for the adjuvant treatment of HER2+ breast cancer [8, 9].
Most women receive trastuzumab in a concurrent treatment schedule. This is largely based on the results from the second NCCTG-N9831 phase-III trial interim-analysis, which showed a better disease free survival (DFS) with a concurrent rather than a sequential schedule (hazard ratio (HR), 0.77; 99.9% confidence interval (CI), 0.53 to 1.11), despite the fact that the results were not statistically significant at the pre-specified boundaries for interim-analysis [1].
NCCTG-N9831 was the only trial directly comparing adjuvant trastuzumab treatment sequences until the recent publication of the combined SIGNAL/PHARE trials [1, 10]. In SIGNAL/PHARE the likelihood of receiving either sequential or concurrent treatment depended on year of inclusion, with a split before and after 2011, the year in which the NCCTG-N9831 interim-analyses was published [10]. To account for this non-random treatment allocation a propensity score methodology was applied. The adjusted comparison showed no significant difference in overall survival (OS)(HR 1.01; 95% CI 0.86–1.19) and DFS (HR 1.08; 95% CI 0.96–1.21) between patients who were treated with a concurrent versus sequential regimen [10].
Both the NCCTG-N9831 interim-analyses and combined SIGNAL/PHARE trials found no significant difference between concurrent and sequential treatment regimens.
The aim of our study was, therefore, to re-evaluate whether there is a difference in outcome between patients who received trastuzumab sequentially after versus concurrently with chemotherapy using real world data from a large, population-based cohort derived from the Netherlands Cancer Registry (NCR), consisting of patients treated prior to the publication of the NCCTG-N9831 results.
Methods
Patient selection
We used the NCR to identify women who were diagnosed in The Netherlands, from January 1st 2005 until but not including January 1st 2008, with a primary HER2+, T1-4NanyM0 breast cancer for which they received any form of both chemotherapy and trastuzumab treatment. Immunohistochemistry for estrogen receptor (ER), progesterone receptor (PR) and HER2 was performed at the local pathology laboratories as part of normal diagnostic workflow. This information was extracted from the pathology reports by NCR datamanagers. ER and PR were considered positive when ≥ 10% of tumor cells stained positive. Tumors were considered HER2 positive when scoring 3 + on immunohistochemistry or showing amplification on in situ hybridization or Multiplex ligation-dependent probe amplification [11,12,13,14].
Information on socio-economic status (SES—low, intermediate, high) was provided to us by the NCR who obtained this information from statistics Netherlands. Statistics Netherlands base their indicator on average income, percentage of people with low income, educational level and unemployment rates at the four digit postal code level of a womans residency at the time of diagnosis [15, 16].
Vital status was obtained through linkage with the municipal population registry. Information on cause of death and the development of subsequent second primary cancers was not available. NCR datamanagers returned to the patient files to retrieve information on disease recurrences as these are not routinely registered in the Dutch cancer registry.
Statistical analysis
Patients were grouped by trastuzumab treatment sequence, concurrent or sequential, based on treatment start- and stopdates. We considered patients to be treated concurrently if they received two or more trastuzumab administration before the end of chemotherapy. All other patients were considered sequentially treated. Differences in baseline characteristics between sequentially and concurrently treated patients were assessed using chi-square, Fisher’s exact and linear-by-linear tests for categorical variables and t-tests for continuous variables.
The endpoints of our study were recurrence free survival (RFS) and OS. RFS time was calculated from date of diagnosis until death from any cause or invasive ipsilateral, local, regional or distant recurrence, whichever occurred first. OS time was calculated from date of diagnosis until death from any cause [17]. Patients lost to follow-up and those without a RFS or OS event were censored at the date of last follow-up.
The Kaplan–Meier method and Cox proportional hazards regression were used to assess RFS and OS. Univariable (unadjusted) and multivariable (adjusted) Cox regression models were used to estimate hazard ratios with 95% confidence intervals to compare treatment groups. We used age, year of diagnosis, grade, pathological T-stage, number of positive lymph nodes, ER-status, PR-status, SES, radiotherapy, hormonal therapy, ovarian ablation and type of chemotherapy received as covariates in our multivariable models. Proportional hazards assumptions were tested for the main, sequential-concurrent treatment effect, using Schoenfeld residuals. The assumptions were satisfied.
Sensitivity analysis were performed using three alternative definitions for concurrent and sequential treatment to check whether using different cut-offs significantly influenced our results. Besides OS and RFS we also calculated distant recurrence free interval (DRFI), defined as distant recurrence or death from breast cancer [17]. Because information on cause of death was lacking in our database we used death following a distant recurrence as a surrogate for death from breast cancer. In addition, due to regional differences in sequential and concurrent treatment, an alternative Cox model incorporating province of residence at time of diagnosis was constructed. Moreso, propensity score matching was performed to reduce possible bias using a nearest neighbor matching approach without replacement in a 1:1 ratio with a caliper of 0.05. Except for chemotherapy, all covariates from the main Cox model were included in a logistic regression model used to obtain propensity scores. Cox models for RFS and OS including matched pair treatment groups were then adjusted for propensity score and chemotherapy. Furthermore, we investigated whether trastuzumab treatment benefit differed by ER-status, nodal status and year of diagnosis, using likelihood ratio testing of interaction terms. Lastly, all analyses were repeated in node positive women and in women who were treated with anthracyclines and taxanes only.
All data were analyzed using R version 3.5.3 and packages ‘coin’, ‘lmtest’, ‘prodlim’, and ‘survival’ [18,19,20,21].
Results
Study population
The NCR identified 2140 potentially eligible women. We excluded 297/2140 (13.9%) women, mostly because of missing treatment start and/or stop dates, precluding classification of the treatment sequence. Hence, 1843 women were included in our study population (Fig. 1).
Baseline characteristics are shown for the entire study population and by trastuzumab treatment sequence (Table 1). In total, 67.0% (1235/1843) of the women in our cohort received trastuzumab concurrently with chemotherapy, while 33.0% (608/1843) received trastuzumab sequentially, following chemotherapy. The proportion of concurrently treated women increased over time from 53.8% (279/519) in 2005 to 76.1% (437/574) in 2007 (P < 0.001, linear-by-linear test) (Table 1, Online Resource 1). Median age at breast cancer diagnosis was 49 years (range 21–74 years) and socio-economic status was medium–high in 71.3% (1314/1843) of women. Most women had tumors that were T2 or smaller (86.5%; 1594/1843), node positive (62.8%; 1158/1843), grade 3 (62.3%; 1149/1843) and ER positive (53.3%; 983/1843) (Table 1). The majority of women received radiotherapy (71.1%; 1310/1843) and endocrine treatment (55.3%; 1020/1843) consisting of tamoxifen, an aromatase inhibitor or one of the two followed by the other. The chemotherapy schedule contained an anthracycline in 91.9% (1694/1843) of all women. In 72.3% (1332/1843) the anthracycline was combined with a taxane, either concurrent or sequential. This treatment approach was used more often in women who received trastuzumab concurrently compared to sequentially, 87.8% (1084/1235) versus 40.8% (248/608), respectively (P < 0.001) (Table 1).
Recurrence free survival
We observed 353 RFS events during a median follow-up of 8.1 years (range 0.3–9.9 years). Of these events, 19.9% (129/608) occurred in sequentially treated women and 18.1% (224/1235) in women who received trastuzumab concurrently with chemotherapy (Table 2). In both groups, distant metastases were the most frequently observed RFS event, followed by local recurrences and death (Online Resource 2). The observed difference in RFS between concurrently and sequentially treated women was not significant (5-year RFS 85.4% versus 83.1%; Plog-rank = 0.2—unadjusted-HR 0.85, 95% CI 0.68–1.06; P = 0.16) (Fig. 2, Table 2). When adjusted for age, year of diagnosis, grade, T-stage, number of positive lymph nodes, ER-status, PR-status, SES, radiotherapy, hormonal therapy, ovarian ablation and the type of chemotherapy received, the HR between concurrent and sequentially treated women remained unchanged (adjusted-HR 0.91, 95% CI 0.67–1.24; P = 0.580) (Table 2).
Overall survival
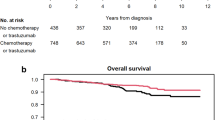
During a median follow-up of 8.2 years (range 0.2–10 years), 286 deaths occurred. Of these events 17.1% (104/608) of deaths occurred in sequentially treated women compared to 14.7% (182/1235) in women who received trastuzumab concurrently with chemotherapy. Again, we found no significant difference between women who received trastuzumab concurrently with chemotherapy when compared to sequentially following chemotherapy (5-year OS 90.2% versus 89.8%; Plog-rank = 0.3—unadjusted-HR 0.87, 95% CI 0.68–1.11; P = 0.269) (Fig. 3, Table 3). When corrected for the abovementioned characteristics the HR for OS between concurrently and sequentially treated women remained unchanged (adjusted-HR 0.92, 95% CI 0.65–1.29; P = 0.635) (Table 3).
Sensitivity analysis
Similar RFS and OS results were obtained in sensitivity analyses using alternative definitions for sequential and concurrent treatment (Online Resource 3). Analyses for DRFI showed 267 events, 89/608 (14.6%) occurring in sequentially treated women compared to 178/1235 (14.4%) women who received trastuzumab concurrently with chemotherapy. The observed difference in DRFI between concurrently and sequentially treated women was not significant (five-year DRFI 87.8% versus 87.2%; Plog-rank = 0.9—djusted-HR 0.96, 95% CI 0.67–1.36; P = 0.833) (Online Resource 4).
We also observed no heterogeneity in the treatment effect by ER-status, nodal status and year of diagnosis for both RFS and OS (all P-values > 0.05). Moreover, adding province as a covariate to our Cox models did not significantly change results (Online Resource 5). Furthermore, when we repeated the analyses excluding 692 women with Nx or N0 disease, the HRs for OS at 5 years (adjusted-HR 0.80 95% CI 0.53–1.20) and RFS (adjusted-HR 0.83 95% CI 0.58–1.17) were comparable to the HR of the main analyses. Likewise, when analyses were repeated, only in women who were treated with anthracyclines and taxanes (n = 1332), HRs for OS (adjusted-HR 0.85 95% CI 0.59–1.21) and RFS (adjusted-HR 0.82 95% CI 0.59–1.13) were similar to the those obtained in the main analyses. Cox models adjusted for propensity scores yielded similar results confirming our main conclusions.
Discussion
In our large population-based cohort of patients with early HER2-positive breast cancer, we found no significant difference in RFS and OS after concurrent versus sequential treatment with chemotherapy and trastuzumab; however, a consistent but non-significant numerical difference in favor of concurrent use was seen for all endpoints.
Most clinical trials that established the role of trastuzumab in the adjuvant setting, compared either the concurrent or sequential chemotherapy-trastuzumab regimen to a control arm containing no trastuzumab [1,2,3,4,5,6,7]. Studies comparing the timing of trastuzumab administration are sparse. The ALTTO study contained sequential and concurrent treatment arms both alone and in combination with lapatinib, but did not directly compare the two treatment sequences [22]. The NCCTG-N9831 trial and combined SIGNAL/PHARE trials are therefore the only trials that compared sequential to concurrent trastuzumab in the adjuvant setting. In the SIGNAL/PHARE trial 5572 women received trastuzumab according to physician’s choice [10], Similar to our study, 65.5% of women in the SIGNAL/PHARE trial were treated concurrently and 34.5% sequentially. After a median follow-up of 58 months, no difference in OS (HR 1.01; 95% CI 0.86–1.19) and DFS (HR 1.08; 95% CI 0.96–1.21) was observed when comparing sequential to concurrent treatment [10]. Results from the NCCTG-N9831 interim-analysis on the other hand are more in line with our results, with a slight improvement in DFS when comparing trastuzumab concurrently with versus sequentially after chemotherapy (HR 0.77; 99.9% CI 0.53–1.11) [1]. The observed difference in DFS was not significant as it did not cross the prespecified O’Brien-Fleming boundary of significance [1]. The definitive joint analysis of the NCCTG-N9831 and NSABP-B31 left out the sequential treatment arm (arm B) from the NCCTG-N9831 trial and compared doxorubicin/cyclophosphamide followed by trastuzumab given concurrently with paclitaxel to a control arm without trastuzumab [23].
Differences between the SIGNAL/PHARE and NCCTG-N9831 studies may be caused by the non-random treatment allocation in the SIGNAL/PHARE study after publication of the NCCTG-N9831 interim-analyses results [10]. However, this was accounted for using a propensity score methodology. Our cohort also observed a significant increase in the proportion of concurrently treated women over time, from 53.8% (279/519) in 2005 to 76.1% (437/574) in 2007 (P < 0.001, linear-by-linear test). Although our cohort originates from before full publication of the NCCTG-N9831 data, its initiation preceeds both the presentation and publication of the first interim-analyses results in 2005 [1, 2, 10].
When looking at the number of women included in our cohort, it seems that there is an imbalance in HER2+ women diagnosed and hence included, with 519 and 750 included women in 2005 and 2006, respectively, compared to 574 women in 2007. However, In the early years of HER2 testing assay quality and interpretation varied considerably between laboratories, leading up to false positive test results in 18% of patients included in large, randomized trials like the NSABP-B31 trial [24]. The variation in number of women included is therefore, most likely a reflection of this high false positive rate rather then a true imbalance in patient inclusion. It wasn’t until 2007 that the American Society for Clinical Oncolgy published a guideline for the recommendation of HER2 testing in breast cancer [25].
Many studies, including SIGNAL/PHARE and the NCCTG-N9831 trial, used DFS as one of their study endpoints. As information on the occurence of second primary cancers was lacking for the women in our cohort we had to use RFS instead [17]. DFS time would have been shorter for women who experienced a second primary cancer in the absence of, or prior to a locoregional or distant recurrence. With a median age of 49 years at diagnosis, however, women in our cohort are relatively young and the incidence of secondary primary cancers low. We therefore think that the results for RFS are comparable to those for DFS.
The concurrent treatment groups in both SIGNAL/PHARE and our study may be enriched with high-risk patients since women received trastuzumab treatment according to physicians choice. However, we observed no variation in baseline characteristics between women who received trstuzumab concurrently with versus sequentially after chemotherapy (Table 1).
Most trials were originally enriched for node positive (N +), high-risk, patients. In our study, 37.2% (685/1843) of the patients were N0. Therefore, we investigated whether trastuzumab sequence benefit differed by nodal status, to ensure that the N0 patients did not influence the observed overall treatment effect. We found no heterogeneity in the treatment effect by nodal status (data not shown).
Anthracyclines are especially effective in HER2-positive breast cancer [26,27,28]. Sequential schedules are preferred as anthracyclines administered concurrently with trastuzumab cause high rates of symptomatic heart failure [28] In the SIGNAL/PHARE trial 33.3% of sequentially treated women received an anthracycline without a taxane compared to 0.8% in the concurrent treatment group [10]. Similarly, in the sequential treatment group of our study 57.1% (348/608) of women received an anthracycline compared to 1.1% (14/1235) in the concurrent treatment group. Since the addition of taxanes to anthracycline-based adjuvant treatment schedules improved the outcome of breast cancer patients in general, regimens for HER2-positive breast cancer patients were developed where trastuzumab was started sequentially after the anthracycline-based part of the regimen and concurrently with a taxane [2, 29, 30]. An alternative strategy was to give six instead of 3–4 anthracycline-based chemotherapy cycles followed sequentially by trastuzumab, which had a low rate of overt heart failure [5]. This may explain why taxanes are given in concurrent treatment groups. In our cohort 1332 women received chemotherapy containing both anthracyclines and taxanes, 1084/1235 (87.8%) concurrently treated women compared to 248/608 (40.8%) sequentially treated women. We repeated the analyses in women who were treated with anthracyclines and taxanes only and found HRs for OS (adjusted-HR 0.85 95% CI 0.53–1.20) and RFS (adjusted-HR 0.83 95% CI 0.58–1.17) that were similar to those obtained in the main analyses meaning that women who received both anthracyclines and taxanes do not derive a differential treatment benefit from trastuzumab treatment sequence.
As cause of death was not known for the women in our cohort we used death following a distant recurrence as a surrogate for death from breast cancer. A substantial number of women in our cohort, therefore, may have died from causes other than breast cancer. In the sequential group 17.8% (23/129) of RFS events consisted of death in the absence of breast cancer recurrence, compared to 12.1% (27/224) in concurrently treated women (Online Resource 2). Although these numbers may seem large, they only pertain to 3.8% (23/608) of the sequentially and 2.2% (27/1235) of the concurrently treated women, respectively. Since neither the clinical SIGNAL/PHARE and NCCTG-N9831 trials nor our population-based study showed superiority of the concurrent over sequential treatment schedule, additional factors like comorbidities and side effects gain importance when choosing a patient’s treatment schedule. The slight imbalance in deaths in the absence of breast cancer recurrence, observed between the sequentially and concurrently treated women in our study, may therefore reflect the clinicians’ preference for a sequential treatment scheme in patients suffering from comorbidities. Unfortunately, we do not have access to reliable information on comorbidities or performance status in our data set to correct for this. We did calculate DRFI to see whether this imbalance impacted outcome and found results similar to the main analyses (Online Resource 3).
In addition, our analysis may have suffered from immortal time bias since only women who did not experience early events, before trastuzumab initiation, were included in our cohort. However, we do not believe that this has impacted our results since there is no reason to believe that the duration of immortal time or the occurrence of early events varies between women who received trastuzumab concurrently with versus sequentially after chemotherapy. In addition, the time between diagnosis and treatment initiation is often relatively short and the incidence of early events low.
Lastly, the results presented in this paper are based on data derived from a population-based cohort. As a result, women were not randomized and received treatment according to the guidelines at time of diagnosis. Although we performed multivariable adjustment for potential confounders, confounding may still play a role in our observational study. Therefore, propensity score matching was performed in an attempt to further reduce any possible confounding effects. The observed change in HR for concurrent versus sequentially treated women, of less than 10% points, was small and therefore confirmed our main conclusions.
Conclusions
In conclusion, although we observed a slight improvement in both OS and RFS in women who received concurrent trastuzumab compared to those treated sequentially, results did not reach statistical significance. Therefore, both treatment approaches are justified and decisions may be made on an individual patient basis where the shorter duration of the concurrent regimen must be balanced with potential treatment-related toxicities and pre-existing comorbidities. A future meta analysis, using all published studies to date, may be useful in providing a more precise estimate of the true difference in outcome between concurrently and sequentially treated women with HER2+ breast cancer.
Data availability
The data that support the findings of this study are available from the Netherlands Cancer Registry, hosted by the Netherlands Comprehensive Cancer Centre (IKNL) but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are, however, available from the authors upon reasonable request and with permission of The Netherlands Comprehensive Cancer Centre (IKNL).
Abbreviations
- A:
-
Doxorubicin
- AI:
-
Aromatase inhibitor
- C:
-
Cyclophosphamide
- Cb:
-
Carboplatin
- CI:
-
Confidence interval
- DFS:
-
Disease free survival
- DRFI:
-
Distant recurrence free interval
- E:
-
Epirubicin
- ER:
-
Estrogen receptor
- F:
-
5-Fluorouracil
- H:
-
Trastuzumab
- HER2:
-
Human Epidermal growth-factor Receptor 2
- HR:
-
Hazard ratio
- LHRH:
-
Luteinizing-hormone-releasing hormone
- NCR:
-
Netherlands Cancer Registry
- N+:
-
Node positive
- OS:
-
Overall survival
- P:
-
Paclitaxel
- PR:
-
Progesterone receptor
- RFS:
-
Recurrence free survival
- SES:
-
Socio-economic status
- T:
-
Docetaxel background
References
Perez EA, Suman VJ, Davidson NE, Gralow JR, Kaufman PA, Visscher DW, Chen B, Ingle JN, Dakhil SR, Zujewski J, Moreno-Aspitia A, Pisansky TM, Jenkins RB (2011) Sequential versus concurrent trastuzumab in adjuvant chemotherapy for breast cancer. J Clin Oncol 29(34):4491–4497. https://doi.org/10.1200/JCO.2011.36.7045
Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE Jr, Davidson NE, Tan-Chiu E, Martino S, Paik S, Kaufman PA, Swain SM, Pisansky TM, Fehrenbacher L, Kutteh LA, Vogel VG, Visscher DW, Yothers G, Jenkins RB, Brown AM, Dakhil SR, Mamounas EP, Lingle WL, Klein PM, Ingle JN, Wolmark N (2005) Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med 353(16):1673–1684. https://doi.org/10.1056/NEJMoa052122
Slamon D, Eiermann W, Robert N, Pienkowski T, Martin M, Press M, Mackey J, Glaspy J, Chan A, Pawlicki M, Pinter T, Valero V, Liu MC, Sauter G, von Minckwitz G, Visco F, Bee V, Buyse M, Bendahmane B, Tabah-Fisch I, Lindsay MA, Riva A, Crown J, Breast Cancer International Research Group (2011) Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med 365(14):1273–1283. https://doi.org/10.1056/NEJMoa0910383
Spielmann M, Roche H, Delozier T, Canon JL, Romieu G, Bourgeois H, Extra JM, Serin D, Kerbrat P, Machiels JP, Lortholary A, Orfeuvre H, Campone M, Hardy-Bessard AC, Coudert B, Maerevoet M, Piot G, Kramar A, Martin AL, Penault-Llorca F (2009) Trastuzumab for patients with axillary-node-positive breast cancer: results of the FNCLCC-PACS 04 trial. J Clin Oncol 27(36):6129–6134. https://doi.org/10.1200/JCO.2009.23.0946
Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, Gianni L, Baselga J, Bell R, Jackisch C, Cameron D, Dowsett M, Barrios CH, Steger G, Huang CS, Andersson M, Inbar M, Lichinitser M, Lang I, Nitz U, Iwata H, Thomssen C, Lohrisch C, Suter TM, Ruschoff J, Suto T, Greatorex V, Ward C, Straehle C, McFadden E, Dolci MS, Gelber RD, Herceptin Adjuvant Trial Study Team (2005) Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med 353(16):1659–1672. https://doi.org/10.1056/NEJMoa052306
Smith I, Procter M, Gelber RD, Guillaume S, Feyereislova A, Dowsett M, Goldhirsch A, Untch M, Mariani G, Baselga J, Kaufmann M, Cameron D, Bell R, Bergh J, Coleman R, Wardley A, Harbeck N, Lopez RI, Mallmann P, Gelmon K, Wilcken N, Wist E, Sanchez Rovira P, Piccart-Gebhart MJ, HS Team (2007) 2-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: a randomised controlled trial. Lancet 369(9555):29–36. https://doi.org/10.1016/S0140-6736(07)60028-2
Gianni L, Dafni U, Gelber RD, Azambuja E, Muehlbauer S, Goldhirsch A, Untch M, Smith I, Baselga J, Jackisch C, Cameron D, Mano M, Pedrini JL, Veronesi A, Mendiola C, Pluzanska A, Semiglazov V, Vrdoljak E, Eckart MJ, Shen Z, Skiadopoulos G, Procter M, Pritchard KI, Piccart-Gebhart MJ, Bell R, Herceptin Adjuvant Trial Study Team (2011) Treatment with trastuzumab for 1 year after adjuvant chemotherapy in patients with HER2-positive early breast cancer: a 4-year follow-up of a randomised controlled trial. Lancet Oncol 12(3):236–244. https://doi.org/10.1016/S1470-2045(11)70033-X
Carlson RW, Allred DC, Anderson BO, Burstein HJ, Carter WB, Edge SB, Erban JK, Farrar WB, Goldstein LJ, Gradishar WJ, Hayes DF, Hudis CA, Jahanzeb M, Kiel K, Ljung BM, Marcom PK, Mayer IA, McCormick B, Nabell LM, Pierce LJ, Reed EC, Smith ML, Somlo G, Theriault RL, Topham NS, Ward JH, Winer EP, Wolff AC, NCCN Breast Cancer Clinical Practice Guidelines Panel (2009) Breast cancer. Clinical practice guidelines in oncology. J Natl Compr Canc Netw 7(2):122–192
The Guideline Database—Initiative of Knowledge Institute of Medical Specialists. https://richtlijnendatabase.nl/en/richtlijn/breast_cancer/adjuvant_systemic_therapy/hormonal_therapy.html (accessed 9 Jan 2018)
Pivot X, Fumoleau P, Pierga JY, Delaloge S, Bonnefoi H, Bachelot T, Jouannaud C, Bourgeois H, Rios M, Soulie P, Jacquin JP, Lavau-Denes S, Kerbrat P, Cox D, Faure-Mercier C, Pauporte I, Gligorov J, Curtit E, Henriques J, Paget-Bailly S, Romieu G (2017) Superimposable outcomes for sequential and concomitant administration of adjuvant trastuzumab in HER2-positive breast cancer: results from the SIGNAL/PHARE prospective cohort. Eur J Cancer 81:151–160. https://doi.org/10.1016/j.ejca.2017.05.020
Cardoso F, Kyriakides S, Ohno S, Penault-Llorca F, Poortmans P, Rubio IT, Zackrisson S, Senkus E, clinicalguidelines@esmo.org EGCEa (2019) Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-updagger. Ann Oncol 30(8):1194–1220. https://doi.org/10.1093/annonc/mdz173
Hammond ME (2011) ASCO-CAP guidelines for breast predictive factor testing: an update. Appl Immunohistochem Mol Morphol 19(6):499–500. https://doi.org/10.1097/PAI.0b013e31822a8eac
Wolff AC, Hammond MEH, Allison KH, Harvey BE, Mangu PB, Bartlett JMS, Bilous M, Ellis IO, Fitzgibbons P, Hanna W, Jenkins RB, Press MF, Spears PA, Vance GH, Viale G, McShane LM, Dowsett M (2018) Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J Clin Oncol 36(20):2105–2122. https://doi.org/10.1200/JCO.2018.77.8738
(IKNL). CCCtN (2020) The Guideline Database - Initiative of Knowledge Institute of Medical Specialists. https://richtlijnendatabase.nl/richtlijn/borstkanker/adjuvante_systemische_therapie.html (accessed 11 May 2020)
Aarts MJ, van der Aa MA, Coebergh JW, Louwman WJ (2010) Reduction of socioeconomic inequality in cancer incidence in the South of the Netherlands during 1996–2008. Eur J Cancer 46(14):2633–2646. https://doi.org/10.1016/j.ejca.2010.07.039
(SCP) SeCP (2019) Socio-economic status according to ZIP code. Sociaal en Cultureel Planbureau (SCP). https://bronnen.zorggegevens.nl/Bron?naam=Sociaal-Economische-Status-per-postcodegebied (accessed 27 Sep 2020)
Hudis CA, Barlow WE, Costantino JP, Gray RJ, Pritchard KI, Chapman JA, Sparano JA, Hunsberger S, Enos RA, Gelber RD, Zujewski JA (2007) Proposal for standardized definitions for efficacy end points in adjuvant breast cancer trials: the STEEP system. J Clin Oncol 25(15):2127–2132. https://doi.org/10.1200/JCO.2006.10.3523
Gerds TA (2017) prodlim: Product-Limit Estimation for Censored Event History Analysis. R package version 1.6.1. https://CRAN.R-project.org/package=prodlim
Torsten ZAH (2002) Diagnostic Checking in Regression Relationships. 2(3). https://CRAN.R-project.org/doc/Rnews/
TM T (2015) A Package for Survival Analysis in S. version 2.38
Hothorn THK, Van de Wiel AM, Zeileis A (2008) implementing a class of permutation tests: the coin package. J Stat Soft. https://doi.org/10.18637/jss.v028.i08
Piccart-Gebhart M, Holmes E, Baselga J, de Azambuja E, Dueck AC, Viale G, Zujewski JA, Goldhirsch A, Armour A, Pritchard KI, McCullough AE, Dolci S, McFadden E, Holmes AP, Tonghua L, Eidtmann H, Dinh P, Di Cosimo S, Harbeck N, Tjulandin S, Im YH, Huang CS, Dieras V, Hillman DW, Wolff AC, Jackisch C, Lang I, Untch M, Smith I, Boyle F, Xu B, Gomez H, Suter T, Gelber RD, Perez EA (2016) Adjuvant lapatinib and trastuzumab for early human epidermal growth factor receptor 2-positive breast cancer: results from the randomized phase III adjuvant lapatinib and/or trastuzumab treatment optimization trial. J Clin Oncol 34(10):1034–1042. https://doi.org/10.1200/JCO.2015.62.1797
Perez EA, Romond EH, Suman VJ, Jeong JH, Sledge G, Geyer CE Jr, Martino S, Rastogi P, Gralow J, Swain SM, Winer EP, Colon-Otero G, Davidson NE, Mamounas E, Zujewski JA, Wolmark N (2014) Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2-positive breast cancer: planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J Clin Oncol 32(33):3744–3752. https://doi.org/10.1200/JCO.2014.55.5730
Paik S, Bryant J, Tan-Chiu E, Romond E, Hiller W, Park K, Brown A, Yothers G, Anderson S, Smith R, Wickerham DL, Wolmark N (2002) Real-world performance of HER2 testing—National Surgical Adjuvant Breast and Bowel Project experience. J Natl Cancer Inst 94(11):852–854. https://doi.org/10.1093/jnci/94.11.852
Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, Dowsett M, Fitzgibbons PL, Hanna WM, Langer A, McShane LM, Paik S, Pegram MD, Perez EA, Press MF, Rhodes A, Sturgeon C, Taube SE, Tubbs R, Vance GH, van de Vijver M, Wheeler TM, Hayes DF, American Society of Clinical Oncology/College of American Pathologists (2007) American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol 25(1):118–145. https://doi.org/10.1200/JCO.2006.09.2775
Muss HB, Thor AD, Berry DA, Kute T, Liu ET, Koerner F, Cirrincione CT, Budman DR, Wood WC, Barcos M et al (1994) c-erbB-2 expression and response to adjuvant therapy in women with node-positive early breast cancer. N Engl J Med 330(18):1260–1266. https://doi.org/10.1056/NEJM199405053301802
Gennari A, Sormani MP, Pronzato P, Puntoni M, Colozza M, Pfeffer U, Bruzzi P (2008) HER2 status and efficacy of adjuvant anthracyclines in early breast cancer: a pooled analysis of randomized trials. J Natl Cancer Inst 100(1):14–20. https://doi.org/10.1093/jnci/djm252
Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L (2001) Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 344(11):783–792. https://doi.org/10.1056/NEJM200103153441101
Henderson IC, Berry DA, Demetri GD, Cirrincione CT, Goldstein LJ, Martino S, Ingle JN, Cooper MR, Hayes DF, Tkaczuk KH, Fleming G, Holland JF, Duggan DB, Carpenter JT, Frei E 3rd, Schilsky RL, Wood WC, Muss HB, Norton L (2003) Improved outcomes from adding sequential Paclitaxel but not from escalating Doxorubicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancer. J Clin Oncol 21(6):976–983. https://doi.org/10.1200/JCO.2003.02.063
Sparano JA, Wang M, Martino S, Jones V, Perez EA, Saphner T, Wolff AC, Sledge GW Jr, Wood WC, Davidson NE (2008) Weekly paclitaxel in the adjuvant treatment of breast cancer. N Engl J Med 358(16):1663–1671. https://doi.org/10.1056/NEJMoa0707056
Tolaney SM, Barry WT, Dang CT, Yardley DA, Moy B, Marcom PK, Albain KS, Rugo HS, Ellis M, Shapira I, Wolff AC, Carey LA, Overmoyer BA, Partridge AH, Guo H, Hudis CA, Krop IE, Burstein HJ, Winer EP (2015) Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer. N Engl J Med 372(2):134–141. https://doi.org/10.1056/NEJMoa1406281
van Ramshorst MS, van der Voort A, van Werkhoven ED, Mandjes IA, Kemper I, Dezentje VO, Oving IM, Honkoop AH, Tick LW, van de Wouw AJ, Mandigers CM, van Warmerdam LJ, Wesseling J, Vrancken Peeters MT, Linn SC, Sonke GS, Dutch Breast Cancer Research Group (2018) Neoadjuvant chemotherapy with or without anthracyclines in the presence of dual HER2 blockade for HER2-positive breast cancer (TRAIN-2): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 19(12):1630–1640. https://doi.org/10.1016/S1470-2045(18)30570-9
van Ramshorst MS, van Werkhoven E, Mandjes IAM, Schot M, Wesseling J, Vrancken Peeters M, Meerum Terwogt JM, Bos MEM, Oosterkamp HM, Rodenhuis S, Linn SC, Sonke GS (2017) Trastuzumab in combination with weekly paclitaxel and carboplatin as neo-adjuvant treatment for HER2-positive breast cancer: the TRAIN-study. Eur J Cancer 74:47–54. https://doi.org/10.1016/j.ejca.2016.12.023
Acknowledgements
The authors would like to thank the Netherlands Comprehensive Cancer Organization for maintaining and collecting information in the Netherlands Cancer Registry and in particular all NCR registrars for the collection and completion of any additional and missing variables for the Netherlands Breast Cancer Project. In addition we would like to thank The Netherlands Organization for Health Research and Development (ZonMW), A Sisters Hope and De Vrienden van UMC Utrecht for their financial support in conducting this study.
Funding
This work was supported by grants from The Netherlands Organization for Health Research and Development [Project Number 836021019]; A Sister’s Hope and De Vrienden van UMC Utrecht. None of the funders had any influence on study design; data collection; and/or project management; data analysis, interpretation; or manuscript preparation, review or approval.
Author information
Authors and Affiliations
Contributions
SCL and GSS conceived the study. The study was designed by GMHED, KJ, MH, SS, GSS and SCL. All data were analyzed by GMHE, KJ and MH and interpreted by all authors. GMHED drafted the manuscript. All authors critically reviewed and approved the manuscript before submission.
Corresponding author
Ethics declarations
Conflict of interest
GSS has received institutional research support funding from AstraZeneca, Merck, Novartis, and Roche outside the submitted work. SCL reports grants from ZonMw and A Sister's Hope during the conduct of the study. SCL is an advisory board member for AstraZeneca, Cergentis, IBM, Pfizer and Roche and received grants from AstraZeneca, Eurocept-pharmaceuticals, Genentech, Novartis, Pfizer, Roche, Tesaro and Immunomedics, in addition, SCL received non-financial support from Genentech, Novartis, Roche, Tesaro and Immunomedics and other from AstraZeneca, Pfizer, Cergentis, IBM and Bayer outside of this study. All remaining authors declare that they have no conflict of interest.
Ethical approval
This project was approved by the Medical Ethical Committee of the Netherlands Cancer Institute – Antoni van Leeuwenhoek hospital (PTC12.1262/NBCP). Data use was approved by the Committee of Privacy of the Netherlands Cancer Registry.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Gabe S. Sonke and Sabine C. Linn contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dackus, G.M.H.E., Jóźwiak, K., van der Wall, E. et al. Concurrent versus sequential use of trastuzumab and chemotherapy in early HER2+ breast cancer. Breast Cancer Res Treat 185, 817–830 (2021). https://doi.org/10.1007/s10549-020-05978-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-020-05978-8