Abstract
Purpose
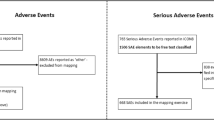
Adverse events (AE) during oncology clinical trials are typically reported using the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE), which provides information about the frequency and severity of AEs from the provider’s perspective. Instruments that track patient-reported outcomes (PRO) complement the CTCAE and provide additional patient-centered information about the toxicity profile of an anti-cancer drug.
Methods
We conducted a single-arm, open-label phase II study of eribulin as first- or second-line therapy for metastatic hormone receptor-positive/HER2-negative (HR+/HER2−) or triple-negative breast cancer (TNBC). Patients were recruited simultaneously into each cohort by tumor subtype. The primary endpoint was overall response rate (ORR). Secondary endpoints included evaluation of toxicity by CTCAE and PRO instruments and agreement between CTCAE and PRO. The study also investigated single-nucleotide polymorphisms (SNPs) associated with treatment-induced neurotoxicity.
Results
83 patients were enrolled: 45 into the HR+/HER2− cohort and 38 into the TNBC cohort. The ORR was 35.6% (90% CI 24–39%) in the HR+/HER2− cohort and 13.2% (90% CI 5–26%) in the TNBC cohort. Stable disease as the best response was recorded in 55.1% of patients with HR+/HER2− disease and 60.5% with TNBC. Toxicity analysis revealed a discordance between CTCAE and PRO assessment in many patients, with a focus on fatigue, alopecia, and neuropathy. Pharmacogenomic analysis identified SNPs associated with treatment-induced peripheral neuropathy.
Conclusions
Eribulin is active in HER2− breast cancer. This study reveals that provider-assessed AEs can vary greatly from patient experiences. Future studies should incorporate CTCAE and PRO instruments to improve reporting of treatment-related AEs.
ClinicalTrials.gov Registration: NCT01827787



Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Trotti A, Colevas AD, Setser A, Rusch V, Jaques D, Budach V, Langer C, Murphy B, Cumberlin R, Coleman CN, Rubin P (2003) CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol 13(3):176–181. https://doi.org/10.1016/S1053-4296(03)00031-6
Trotti A, Colevas AD, Setser A, Basch E (2007) Patient-reported outcomes and the evolution of adverse event reporting in oncology. J Clin Oncol 25(32):5121–5127. https://doi.org/10.1200/JCO.2007.12.4784
Basch E, Deal AM, Kris MG, Scher HI, Hudis CA, Sabbatini P, Rogak L, Bennett AV, Dueck AC, Atkinson TM, Chou JF, Dulko D, Sit L, Barz A, Novotny P, Fruscione M, Sloan JA, Schrag D (2016) Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol 34(6):557–565. https://doi.org/10.1200/jco.2015.63.0830
Basch E, Deal AM, Dueck AC, Scher HI, Kris MG, Hudis C, Schrag D (2017) Overall survival results of a trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment. JAMA 318(2):197–198. https://doi.org/10.1001/jama.2017.7156
Basch E, Reeve BB, Mitchell SA, Clauser SB, Minasian LM, Dueck AC, Mendoza TR, Hay J, Atkinson TM, Abernethy AP, Bruner DW, Cleeland CS, Sloan JA, Chilukuri R, Baumgartner P, Denicoff A, St Germain D, O'Mara AM, Chen A, Kelaghan J, Bennett AV, Sit L, Rogak L, Barz A, Paul DB, Schrag D (2014) Development of the National Cancer Institute's patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). J Natl Cancer Inst 106(9):244. https://doi.org/10.1093/jnci/dju244
Dueck AC, Mendoza TR, Mitchell SA, Reeve BB, Castro KM, Rogak LJ, Atkinson TM, Bennett AV, Denicoff AM, O'Mara AM, Li Y, Clauser SB, Bryant DM, Bearden JD 3rd, Gillis TA, Harness JK, Siegel RD, Paul DB, Cleeland CS, Schrag D, Sloan JA, Abernethy AP, Bruner DW, Minasian LM, Basch E, National Cancer Institute PROCSG (2015) Validity and reliability of the US National Cancer Institute's patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). JAMA Oncol 1(8):1051–1059. https://doi.org/10.1001/jamaoncol.2015.2639
Thanarajasingam G, Atherton PJ, Novotny PJ, Loprinzi CL, Sloan JA, Grothey A (2016) Longitudinal adverse event assessment in oncology clinical trials: the toxicity over time (ToxT) analysis of alliance trials NCCTG N9741 and 979254. Lancet Oncol 17(5):663–670. https://doi.org/10.1016/S1470-2045(16)00038-3
Cortes J, O'Shaughnessy J, Loesch D, Blum JL, Vahdat LT, Petrakova K, Chollet P, Manikas A, Dieras V, Delozier T, Vladimirov V, Cardoso F, Koh H, Bougnoux P, Dutcus CE, Seegobin S, Mir D, Meneses N, Wanders J, Twelves C, Investigators E (2011) Eribulin monotherapy versus treatment of physician's choice in patients with metastatic breast cancer (EMBRACE): a phase 3 open-label randomised study. Lancet 377(9769):914–923. https://doi.org/10.1016/S0140-6736(11)60070-6
Kaufman PA, Awada A, Twelves C, Yelle L, Perez EA, Velikova G, Olivo MS, He Y, Dutcus CE, Cortes J (2015) Phase III open-label randomized study of eribulin mesylate versus capecitabine in patients with locally advanced or metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol 33(6):594–601. https://doi.org/10.1200/JCO.2013.52.4892
McIntyre K, O'Shaughnessy J, Schwartzberg L, Gluck S, Berrak E, Song JX, Cox D, Vahdat LT (2014) Phase 2 study of eribulin mesylate as first-line therapy for locally recurrent or metastatic human epidermal growth factor receptor 2-negative breast cancer. Breast Cancer Res Treat 146(2):321–328. https://doi.org/10.1007/s10549-014-2923-9
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45(2):228–247. https://doi.org/10.1016/j.ejca.2008.10.026
Brady MJ, Cella DF, Mo F, Bonomi AE, Tulsky DS, Lloyd SR, Deasy S, Cobleigh M, Shiomoto G (1997) Reliability and validity of the functional assessment of cancer therapy-breast quality-of-life instrument. J Clin Oncol 15(3):974–986. https://doi.org/10.1200/JCO.1997.15.3.974
Cella D, Wang M, Wagner L, Miller K (2011) Survival-adjusted health-related quality of life (HRQL) among patients with metastatic breast cancer receiving paclitaxel plus bevacizumab versus paclitaxel alone: results from Eastern Cooperative Oncology Group Study 2100 (E2100). Breast Cancer Res Treat 130(3):855–861. https://doi.org/10.1007/s10549-011-1725-6
Calhoun EA, Welshman EE, Chang CH, Lurain JR, Fishman DA, Hunt TL, Cella D (2003) Psychometric evaluation of the Functional Assessment of Cancer Therapy/Gynecologic Oncology Group-Neurotoxicity (Fact/GOG-Ntx) questionnaire for patients receiving systemic chemotherapy. Int J Gynecol Cancer 13(6):741–748. https://doi.org/10.1111/j.1525-1438.2003.13603.x
Dueck AC, Mendoza TR, Mitchell SA, Reeve BB, Castro KM, Denicoff A, O'Mara AM, Rogak LJ, Clauser SB, Bryant DM, Gillis TA, Bearden JD, Siegel RD, Harness JK, Paul DB, Cleeland CS, Sloan JA, Schrag D, Minasian LM, Basch EM (2012) Validity and reliability of the patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). J Clin Oncol 30(15_suppl):9047–9047. https://doi.org/10.1200/jco.2012.30.15_suppl.9047
Quinten C, Maringwa J, Gotay CC, Martinelli F, Coens C, Reeve BB, Flechtner H, Greimel E, King M, Osoba D, Cleeland C, Ringash J, Schmucker-Von Koch J, Taphoorn MJ, Weis J, Bottomley A (2011) Patient self-reports of symptoms and clinician ratings as predictors of overall cancer survival. J Natl Cancer Inst 103(24):1851–1858. https://doi.org/10.1093/jnci/djr485
Schneider BP, Li L, Miller K, Flockhart D, Radovich M, Hancock BA, Kassem N, Foroud T, Koller DL, Badve SS, Li Z, Partridge AH, O'Neill AM, Sparano JA, Dang CT, Northfelt DW, Smith ML, Railey E, Sledge GW (2011) Genetic associations with taxane-induced neuropathy by a genome-wide association study (GWAS) in E5103. J Clin Oncol 29(15_Suppl):1000–1000
Pakhomov SV, Jacobsen SJ, Chute CG, Roger VL (2008) Agreement between patient-reported symptoms and their documentation in the medical record. Am J Manage Care 14(8):530–539
Schwartzberg L, McIntyre K, Wilks S, Puhalla S, O'Shaughnessy J, Berrak E, He Y, Vahdat L (2019) Health-related quality of life in patients receiving first-line eribulin mesylate with or without trastuzumab for locally recurrent or metastatic breast cancer. BMC Cancer 19(1):578. https://doi.org/10.1186/s12885-019-5674-5
Sacks CA, Miller PW, Longo DL (2019) Talking about toxicity—"what we've got here is a failure to communicate". N Engl J Med 381(15):1406–1408. https://doi.org/10.1056/NEJMp1908310
Fiero MH, Roydhouse JK, Vallejo J, King-Kallimanis BL, Kluetz PG (2017) Sridhara R (2019) US Food and Drug Administration review of statistical analysis of patient-reported outcomes in lung cancer clinical trials approved between January, 2008, and December. Lancet Oncol 20(10):e582–e589. https://doi.org/10.1016/S1470-2045(19)30335-3
Tolaney SM et al (2017) Phase 1b/2 study to evaluate eribulin mesylate in combination with pembrolizumab in patients with metastatic triple-negative breast cancer. In: Paper presented at the San Antonio Breast Cancer Symposium, San Antonio
Baldwin RM, Owzar K, Zembutsu H, Chhibber A, Kubo M, Jiang C, Watson D, Eclov RJ, Mefford J, McLeod HL, Friedman PN, Hudis CA, Winer EP, Jorgenson EM, Witte JS, Shulman LN, Nakamura Y, Ratain MJ, Kroetz DL (2012) A genome-wide association study identifies novel loci for paclitaxel-induced sensory peripheral neuropathy in CALGB 40101. Clin Cancer Res 18(18):5099–5109. https://doi.org/10.1158/1078-0432.CCR-12-1590
Acknowledgements
Timothy K. Erick, PhD, a full-time medical writer employed by Dana-Farber Cancer Institute, assisted in the editing and preparation of the final manuscript. Kaitlyn Bifolck, B.A., a full-time editor employed by Dana-Farber Cancer Institute, assisted in the editing, preparation, and submission of the final manuscript.
Funding
Supported in part by an investigator-initiated grant from Eisai, Inc.
Author information
Authors and Affiliations
Contributions
OMF: conceptualization, data curation, formal analysis, investigation, methodology, resources, validation, writing—original draft, and writing—review and editing. AG-H: conceptualization, data curation, formal analysis, investigation, methodology, resources, software, writing—original draft, and writing—review and editing. NUL: data curation, investigation, writing—review and editing. MF: data curation, investigation, writing—review and editing. SC: data curation, investigation, writing—review and editing. TO: data curation, investigation, writing—review and editing. MC: data curation, investigation, writing—review and editing. JW: data curation, investigation, writing—review and editing. RF: data curation, investigation, writing—review and editing. BS: data curation, formal analysis, investigation, methodology, software, writing—review and editing. HJB: conceptualization, data curation, investigation, writing—original draft, and writing—review and editing. ELM: conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, resources, validation, writing—original draft, and writing—review and editing.
Corresponding author
Ethics declarations
Conflict of interest
O. Metzger Filho reports research funding to institution from Abbvie, Cascadian Therapeutics, Eisai, Pfizer, Roche/Genentech, and Susan G. Komen for the Cure; consulting/advisory roles for Abbvie, G1 Therapeutics, and Groupo Oncoclinicas (Brazil); and honoraria from Roche (Brazil); travel/accommodations/expenses from Grupo Oncoclinicas. N.U. Lin reports research funding to institution from Seattle Genetics, Genentech, Pfizer, Merck; advisory board roles for Daichii Sankyo, Puma, Seattle Genetics; and royalties from UpToDate. R. Freedman reports research funding to institution from Eisai and Puma. E.L. Mayer reports research funding to institution from Pfizer, Eisai. Advisory Board: Lilly, Novartis, and Eisai. All other authors report no disclosures.
Ethical approval
This trial was approved by the individual institutional review boards at participating cancer centers and conducted according to the provisions of the Declaration of Helsinki and Good Clinical Practice.
Informed consent
Informed consent was obtained from all individual participants included in the study. All patients provided written informed consent before any study-related procedures.
Research involving human participants
This trial was approved by the individual institutional review boards at participating cancer centers and conducted according to the provisions of the Declaration of Helsinki and Good Clinical Practice.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Filho, O.M., Giobbie-Hurder, A., Lin, N.U. et al. A dynamic portrait of adverse events for breast cancer patients: results from a phase II clinical trial of eribulin in advanced HER2-negative breast cancer. Breast Cancer Res Treat 185, 135–144 (2021). https://doi.org/10.1007/s10549-020-05928-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-020-05928-4