Abstract
Purpose
Non-adherence to the oral anti-estrogen therapies (AET) tamoxifen and aromatase inhibitors in early-stage hormone receptor-positive breast cancer is associated with numerous negative clinical outcomes. Prior studies have identified that non-adherence is associated with psychological and menopause-related factors which are present during AET, but the presence of these characteristics prior to AET initiation has not been investigated.
Methods
Psychological and menopause symptoms (depression, generalized anxiety, insomnia, somatosensory amplification, hot flash frequency, and hot flash-related interference) were assessed pre-AET initiation as predictors of subsequent non-adherence in 73 participants (Mage = 55.0, SD = 10.1 years). Participants self-reported treatment adherence after three and 6 weeks on AET. Participants who did not initiate treatment were excluded from the analysis.
Results
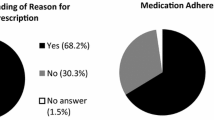
Discriminant function analyses revealed that the hypothesized set of psychological and menopause symptoms at baseline (pre-AET) together statistically distinguished between those who were non-adherent (n = 19; 26.0%) from adherent (n = 54; 74.0%) at 6 weeks. Model classification accuracy was statistically significant (Wilks’ ƛ = 0.782, χ2(6) = 15.50, p = 0.017) at the 6-week timepoint. Results were consistent at 3 weeks. Pre-AET psychological and menopause symptoms correctly classified 6-week treatment adherence 77.9% of the time. Depression contributed most to distinguishing between adherers and non-adherers.
Conclusions
The presence of a composite profile of psychological and menopause symptoms prior to AET initiation may help to identify early treatment non-adherence. Results can be used to identify patients at risk for non-adherence and to guide psychological and symptom management interventions.

Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- AET:
-
Anti-estrogen therapies
- DFA:
-
Discriminant Function Analysis
- GAD-7:
-
Generalized Anxiety Disorder
- HFRDIS:
-
Hot Flash-Related Daily Interference Scale
- ISI:
-
Insomnia Severity Index
- PHQ-8:
-
Patient Health Questionnaire
- SSAS:
-
Somatosensory Amplification
References
Burstein HJ, Temin S, Anderson H et al (2014) Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: American Society of Clinical Oncology clinical practice guideline focused update. J Clin Oncol. https://doi.org/10.1200/JCO.2013.54.2258
McCowan C, Shearer J, Donnan PT et al (2008) Cohort study examining tamoxifen adherence and its relationship to mortality in women with breast cancer. Br J Cancer. https://doi.org/10.1038/sj.bjc.6604758
Hershman DL, Kushi LH, Shao T et al (2010) Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients. J Clin Oncol. https://doi.org/10.1200/JCO.2009.25.9655
Hershman DL, Kushi LH, Hillyer GC et al (2016) Psychosocial factors related to non-persistence with adjuvant endocrine therapy among women with breast cancer: the Breast Cancer Quality of Care Study (BQUAL). Breast Cancer Res Treat. https://doi.org/10.1007/s10549-016-3788-x
He W, Fang F, Varnum C, Eriksson M, Hall P, Czene K (2015) Predictors of discontinuation of adjuvant hormone therapy in patients with breast cancer. J Clin Oncol 33(20):2262–2269. https://doi.org/10.1200/JCO.2014.59.3673
Murphy CC, Bartholomew LK, Carpentier MY, Bluethmann SM, Vernon SW (2012) Adherence to adjuvant hormonal therapy among breast cancer survivors in clinical practice: a systematic review. Breast Cancer Res Treat. https://doi.org/10.1007/s10549-012-2114-5
Moon Z, Moss-Morris R, Hunter MS, Carlisle S, Hughes LD (2017) Barriers and facilitators of adjuvant hormone therapy adherence and persistence in women with breast cancer: a systematic review. Patient Prefer Adherence. https://doi.org/10.2147/PPA.S126651
Owusu C, Buist DSM, Field TS et al (2008) Predictors of tamoxifen discontinuation among older women with estrogen receptor-positive breast cancer. J Clin Oncol Off J Am Soc Clin Oncol 26(4):549–555. https://doi.org/10.1200/JCO.2006.10.1022
Grunfeld EA, Hunter MS, Sikka P, Mittal S (2005) Adherence beliefs among breast cancer patients taking tamoxifen. Patient Educ Couns. https://doi.org/10.1016/j.pec.2004.10.005
Ell K, Vourlekis B, Xie B et al (2009) Cancer treatment adherence among low-income women with breast or gynecologic cancer: a randomized controlled trial of patient navigation. Cancer. https://doi.org/10.1002/cncr.24500
Sheppard VB, de Mendoza AH, He J et al (2018) Initiation of adjuvant endocrine therapy in black and white women with breast cancer. Clin Breast Cancer 18(5):337–346.e1. https://doi.org/10.1016/j.clbc.2017.12.002
Kahn KL, Schneider EC, Malin JL, Adams JL, Epstein AM (2007) Patient centered experiences in breast cancer: predicting long-term adherence to tamoxifen use. Med Care 45(5):431–439. https://doi.org/10.1097/01.mlr.0000257193.10760.7f
Chlebowski RT, Kim J, Haque R (2014) Adherence to endocrine therapy in breast cancer adjuvant and prevention settings. Cancer Prev Res (Phila) 7(4):378–387. https://doi.org/10.1158/1940-6207.CAPR-13-0389
Lambert LK, Balneaves LG, Howard AF, Gotay CC (2018) Patient-reported factors associated with adherence to adjuvant endocrine therapy after breast cancer: an integrative review. Breast Cancer Res Treat 167(3):615–633. https://doi.org/10.1007/s10549-017-4561-5
Simon R, Latreille J, Matte C, Desjardins P, Bergeron E (2014) Adherence to adjuvant endocrine therapy in estrogen receptor-positive breast cancer patients with regular follow-up. Can J Surg 57(1):26–32. https://doi.org/10.1503/cjs.006211
Hadji P, Ziller V, Kyvernitakis J et al (2013) Persistence in patients with breast cancer treated with tamoxifen or aromatase inhibitors: a retrospective database analysis. Breast Cancer Res Treat 138(1):185–191. https://doi.org/10.1007/s10549-013-2417-1
Lam WY, Fresco P (2015) Medication adherence measures: an overview. Giardini A (ed.). Biomed Res Int. 2015:217047. https://doi.org/10.1155/2015/217047
Wassermann J, Gelber SI, Rosenberg SM et al (2019) Nonadherent behaviors among young women on adjuvant endocrine therapy for breast cancer. Cancer 125(18):3266–3274. https://doi.org/10.1002/cncr.32192
Kroenke K, Strine TW, Spitzer RL, Williams JBW, Berry JT, Mokdad AH (2009) The PHQ-8 as a measure of current depression in the general population. J Affect Disord 114(1):163–173. https://doi.org/10.1016/j.jad.2008.06.026
Reyes-Gibby CC, Anderson KO, Morrow PK, Shete S, Hassan S (2011) Depressive symptoms and health-related quality of life in breast cancer survivors. J Women’s Heal 21(3):311–318. https://doi.org/10.1089/jwh.2011.2852
Barsky AJ, Wyshak G, Klerman GL (1990) The somatosensory amplification scale and its relationship to hypochondriasis. J Psychiatr Res 24(4):323–334. https://doi.org/10.1016/0022-3956(90)90004-A
Mausbach BT, Schwab RB, Irwin SA (2015) Depression as a predictor of adherence to adjuvant endocrine therapy (AET) in women with breast cancer: a systematic review and meta-analysis. Breast Cancer Res Treat 152(2):239–246. https://doi.org/10.1007/s10549-015-3471-7
Reece JC, Chan Y-F, Herbert J, Gralow J, Fann JR (2013) Course of depression, mental health service utilization and treatment preferences in women receiving chemotherapy for breast cancer. Gen Hosp Psychiatry 35(4):376–381. https://doi.org/10.1016/j.genhosppsych.2013.03.017
Bastien CH, Vallières A, Morin CM (2001) Validation of the insomnia severity index as an outcome measure for insomnia research. Sleep Med 2(4):297–307. https://doi.org/10.1016/S1389-9457(00)00065-4
Savard MH, Savard J, Simard S, Ivers H (2005) Empirical validation of the insomnia severity index in cancer patients. Psychooncology. https://doi.org/10.1002/pon.860
Yusufov M, Zhou ES, Recklitis CJ (2019) Psychometric properties of the Insomnia Severity Index in cancer survivors. Psychooncology 28(3):540–546. https://doi.org/10.1002/pon.4973
Newton KM, Carpenter JS, Guthrie KA et al (2014) Methods for the design of vasomotor symptom trials. Menopause. https://doi.org/10.1097/gme.0b013e31829337a4
Carpenter JS (2001) The hot flash related daily interference scale: a tool for assessing the impact of hot flashes on quality of life following breast cancer. J Pain Symptom Manage 22(6):979–989. https://doi.org/10.1016/S0885-3924(01)00353-0
Rencher AC (1992) Interpretation of canonical discriminant functions, canonical variates, and principal components. Am Stat. https://doi.org/10.2307/2685219
Meyers LS, Gamst G, Guarino AJ (2006) Applied multivariate research: design and interpretation. Sage publications, Thousand Oaks
Bujang MA, Saat N, Sidik TMITAB, Joo LC (2018) Sample size guidelines for logistic regression from observational studies with large population: emphasis on the accuracy between statistics and parameters based on real life clinical data. Malays J Med Sci 25(4):122–130
Martínez MP, Belloch A, Botella C (1999) Somatosensory amplification in hypochondriasis and panic disorder. Clin Psychol Psychother 6(1):46–53
Barsky AJ (2001) The patient with hypochondriasis. N Engl J Med 345(19):1395–1399. https://doi.org/10.1056/NEJMcp002896
Paranjpe R, John G, Trivedi M, Abughosh S (2019) Identifying adherence barriers to oral endocrine therapy among breast cancer survivors. Breast Cancer Res Treat 174(2):297–305. https://doi.org/10.1007/s10549-018-05073-z
Nestoriuc Y, von Blanckenburg P, Schuricht F et al (2016) Is it best to expect the worst? Influence of patients’ side-effect expectations on endocrine treatment outcome in a 2-year prospective clinical cohort study. Ann Oncol 27(10):1909–1915. https://doi.org/10.1093/annonc/mdw266
Brett J, Fenlon D, Boulton M et al (2018) Factors associated with intentional and unintentional non-adherence to adjuvant endocrine therapy following breast cancer. Eur J Cancer Care (Engl) 27(1):1–20. https://doi.org/10.1111/ecc.12601
Rosenberg SM, Stanton AL, Petrie KJ, Partridge AH (2015) Symptoms and symptom attribution among women on endocrine therapy for breast cancer. Oncologist 20(6):598–604. https://doi.org/10.1634/theoncologist.2015-0007
Duddu V, Isaac MK, Chaturvedi SK (2006) Somatization, somatosensory amplification, attribution styles and illness behaviour: a review. Int Rev Psychiatry 18(1):25–33. https://doi.org/10.1080/09540260500466790
Bowles EJA, Boudreau DM, Chubak J et al (2012) Patient-reported discontinuation of endocrine therapy and related adverse effects among women with early-stage breast cancer. J Oncol Pract 8(6):149–157. https://doi.org/10.1200/JOP.2012.000543
Kidwell KM, Harte SE, Hayes DF et al (2014) Patient-reported symptoms and discontinuation of adjuvant aromatase inhibitor therapy. Cancer 120(16):2403–2411. https://doi.org/10.1002/cncr.28756
Lash TL, Fox MP, Westrup JL, Fink AK, Silliman RA (2006) Adherence to tamoxifen over the five-year course. Breast Cancer Res Treat 99(2):215–220. https://doi.org/10.1007/s10549-006-9193-0
Stanton AL, Petrie KJ, Partridge AH (2014) Contributors to nonadherence and nonpersistence with endocrine therapy in breast cancer survivors recruited from an online research registry. Breast Cancer Res Treat 145(2):525–534. https://doi.org/10.1007/s10549-014-2961-3
Garber MC, Nau DP, Erickson SR, Aikens JE, Lawrence JB (2004) The concordance of self-report with other measures of medication adherence: a summary of the literature. Med Care 42(7):649–652. https://doi.org/10.1097/01.mlr.0000129496.05898.02
Nemeroff CB (2002) Comorbidity of mood and anxiety disorders: the rule, not the exception? Am J Psychiatry 159(1):3–4. https://doi.org/10.1176/appi.ajp.159.1.3
Spaan P, van Luenen S, Garnefski N, Kraaij V (2018) Psychosocial interventions enhance HIV medication adherence: A systematic review and meta-analysis. J Health Psychol. https://doi.org/10.1177/1359105318755545
Cramer JA (2004) A systematic review of adherence with. Diabetes Care 27:1218–1224. https://doi.org/10.2337/diacare.27.5.1218
Martin LR, Williams SL, Haskard KB, Dimatteo MR (2005) The challenge of patient adherence. Ther Clin Risk Manag 1(3):189–199
Spitzer RL, Kroenke K, Williams JBW, Löwe B (2006) A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med 166(10):1092–1097. https://doi.org/10.1001/archinte.166.10.1092
Acknowledgements
This work was conducted with support from the Harvard Catalyst | The Harvard Clinical and Translational Science Center (National Center for Advancing Translational Sciences, National Institutes of Health Award UL 1TR002541) and financial contributions from Harvard University and its affiliated academic healthcare centers. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University, and its affiliated academic healthcare centers, or the National Institutes of Health. This research was supported by the Brigham and Women’s Hospital Department of Psychiatry and the Dana-Farber Cancer Institute Department of Psychosocial Oncology and Palliative Care Research Fellowship.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Data analysis was performed by MY and AW. The first draft of the manuscript was written by MY and MN and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Dr. Miryam Yusufov reports in the past 12 months: Consultant: Blue Note Therapeutics. Dr. Hadine Joffe reports in past 12 months: Consultant/Advisory Board: NeRRe/KaNDy, Sojournix, Eisai. Grant/Research Support: National Institutes of Health, Merck, NeRRe/KaNDy, Pfizer, QUE Oncology. Spouse: Merck Research Lab—employee; Arsenal Biosciences—consulting and equity, Tango—equity.
Ethical approval
All participants provided written informed consent for study procedures, which were approved by the Institutional Review Board of Dana-Farber/Harvard Cancer Center (Protocol # 14-206).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yusufov, M., Nathan, M., Wiley, A. et al. Predictors of increased risk for early treatment non-adherence to oral anti-estrogen therapies in early-stage breast cancer patients. Breast Cancer Res Treat 185, 53–62 (2021). https://doi.org/10.1007/s10549-020-05920-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-020-05920-y