Abstract
Purpose
Neoadjuvant clinical trials with dual HER2 blockade with pertuzumab and trastuzumab plus chemotherapy demonstrated high rates of pathological complete response (pCR) in HER2-positive early breast cancer (BC). We investigated whether the benefit on pCR seen in clinical trials is confirmed in a real-world setting.
Methods
Multicenter, retrospective study in patients with HER2-positive early BC receiving neoadjuvant treatment with pertuzumab and trastuzumab in routine clinical practice (n = 243). The primary endpoint was total pCR (tpCR) (ypT0/is ypN0).
Results
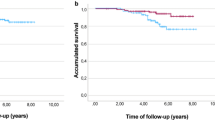
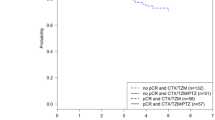
A total of 243 evaluable patients were included. Pertuzumab and trastuzumab were combined with anthracyclines and taxanes in 74.1% of patients, with single-agent taxane in 11.1% of patients and with platinum-based chemotherapy (CT) in 14.4% of patients. The tpCR rate was 66.4%:71% with anthracyclines and taxanes, 59.3% with single-agent taxane, and 48.6% with platinum-based combinations. The tpCR rate was higher among patients with hormone receptor (HR)-negative tumors (80.9%) vs HR-positive tumors (55.4%) (p < 0.001). A pCR in the breast (ypT0/is) was achieved in 67.6% of patients. Of 143 patients who showed radiological complete response (rCR) (62%), 112 (78.3%) patients also achieved tpCR. Assessment of rCR by magnetic resonance imaging (MRI) showed the highest negative predictive value (NPV) for predicting tpCR (83.5%). Breast-conserving surgery was performed in 58.7% of patients. Grade 3 and grade 4 toxicities were reported in 33 (18.2%) and 12 (6.6%) patients, respectively. No toxicity leading to death was reported.
Conclusions
This real-world analysis shows that neoadjuvant pertuzumab, trastuzumab, and chemotherapy achieve comparable or even higher rates of tpCR than those seen in clinical trials. The pCR benefit is higher in HR-negative tumors. The assessment of rCR by MRI showed the highest ability for predicting pCR. In addition, this neoadjuvant strategy confers an acceptable safety profile.
Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request. Qualified researchers may request access to individual patient-level data through the clinical study data request platform (https://vivli.org/). Further details on Roche's criteria for eligible studies are available here (https://vivli.org/members/ourmembers/). For further details on Roche's Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see here: (https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm).”
References
Owens MA, Horten BC, Da Silva MM (2004) HER2 amplification ratios by fluorescence in situ hybridization and correlation with immunohistochemistry in a cohort of 6556 breast cancer tissues. Clin Breast Cancer 5(1):63–69
Ross JS, Slodkowska EA, Symmans WF, Pusztai L, Ravdin PM, Hortobagyi GN (2009) The HER-2 receptor and breast cancer: ten years of targeted anti-HER-2 therapy and personalized medicine. Oncologist 14(4):320–368. https://doi.org/10.1634/theoncologist.2008-0230
Menard S, Tagliabue E, Campiglio M, Pupa SM (2000) Role of HER2 gene overexpression in breast carcinoma. J Cell Physiol 182(2):150–162. https://doi.org/10.1002/(SICI)1097-4652(200002)182:2<150:AID-JCP3>3.0.CO;2-E
Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL (1987) Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 235(4785):177–182
Chen AM, Meric-Bernstam F, Hunt KK, Thames HD, Oswald MJ, Outlaw ED, Strom EA, McNeese MD, Kuerer HM, Ross MI, Singletary SE, Ames FC, Feig BW, Sahin AA, Perkins GH, Schechter NR, Hortobagyi GN, Buchholz TA (2004) Breast conservation after neoadjuvant chemotherapy: the MD Anderson cancer center experience. J Clin Oncol 22(12):2303–2312. https://doi.org/10.1200/JCO.2004.09.062
Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, Bonnefoi H, Cameron D, Gianni L, Valagussa P, Swain SM, Prowell T, Loibl S, Wickerham DL, Bogaerts J, Baselga J, Perou C, Blumenthal G, Blohmer J, Mamounas EP, Bergh J, Semiglazov V, Justice R, Eidtmann H, Paik S, Piccart M, Sridhara R, Fasching PA, Slaets L, Tang S, Gerber B, Geyer CE Jr, Pazdur R, Ditsch N, Rastogi P, Eiermann W, von Minckwitz G (2014) Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 384(9938):164–172. https://doi.org/10.1016/S0140-6736(13)62422-8
Prowell TM, Pazdur R (2012) Pathological complete response and accelerated drug approval in early breast cancer. N Engl J Med 366(26):2438–2441. https://doi.org/10.1056/NEJMp1205737
Symmans WF, Wei C, Gould R, Yu X, Zhang Y, Liu M, Walls A, Bousamra A, Ramineni M, Sinn B, Hunt K, Buchholz TA, Valero V, Buzdar AU, Yang W, Brewster AM, Moulder S, Pusztai L, Hatzis C, Hortobagyi GN (2017) Long-term prognostic risk after neoadjuvant chemotherapy associated with residual cancer burden and breast cancer subtype. J Clin Oncol 35(10):1049–1060. https://doi.org/10.1200/JCO.2015.63.1010
Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE Jr, Davidson NE, Tan-Chiu E, Martino S, Paik S, Kaufman PA, Swain SM, Pisansky TM, Fehrenbacher L, Kutteh LA, Vogel VG, Visscher DW, Yothers G, Jenkins RB, Brown AM, Dakhil SR, Mamounas EP, Lingle WL, Klein PM, Ingle JN, Wolmark N (2005) Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med 353(16):1673–1684. https://doi.org/10.1056/NEJMoa052122
Gianni L, Eiermann W, Semiglazov V, Manikhas A, Lluch A, Tjulandin S, Zambetti M, Vazquez F, Byakhow M, Lichinitser M, Climent MA, Ciruelos E, Ojeda B, Mansutti M, Bozhok A, Baronio R, Feyereislova A, Barton C, Valagussa P, Baselga J (2010) Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet 375(9712):377–384. https://doi.org/10.1016/S0140-6736(09)61964-4
Cardoso F, Piccart MJ, Durbecq V, Di Leo A (2002) Resistance to trastuzumab: a necessary evil or a temporary challenge? Clin Breast Cancer 3(4):247–257. discussion 258–249. https://doi.org/10.3816/CBC.2002.n.028
Gallardo A, Lerma E, Escuin D, Tibau A, Munoz J, Ojeda B, Barnadas A, Adrover E, Sanchez-Tejada L, Giner D, Ortiz-Martinez F, Peiro G (2012) Increased signalling of EGFR and IGF1R, and deregulation of PTEN/PI3K/Akt pathway are related with trastuzumab resistance in HER2 breast carcinomas. Br J Cancer 106(8):1367–1373. https://doi.org/10.1038/bjc.2012.85
Franklin MC, Carey KD, Vajdos FF, Leahy DJ, de Vos AM, Sliwkowski MX (2004) Insights into ErbB signaling from the structure of the ErbB2-pertuzumab complex. Cancer Cell 5(4):317–328
Gianni L, Pienkowski T, Im YH, Roman L, Tseng LM, Liu MC, Lluch A, Staroslawska E, de la Haba-Rodriguez J, Im SA, Pedrini JL, Poirier B, Morandi P, Semiglazov V, Srimuninnimit V, Bianchi G, Szado T, Ratnayake J, Ross G, Valagussa P (2012) Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol 13(1):25–32. https://doi.org/10.1016/S1470-2045(11)70336-9
Loibl S, Jackisch C, Schneeweiss A, Schmatloch S, Aktas B, Denkert C, Wiebringhaus H, Kummel S, Warm M, Paepke S, Just M, Hanusch C, Hackmann J, Blohmer JU, Clemens M, Dan Costa S, Gerber B, Engels K, Nekljudova V, von Minckwitz G, Untch M (2017) Dual HER2-blockade with pertuzumab and trastuzumab in HER2-positive early breast cancer: a subanalysis of data from the randomized phase III GeparSepto trial. Ann Oncol 28(3):497–504. https://doi.org/10.1093/annonc/mdw610
Schneeweiss A, Chia S, Hickish T, Harvey V, Eniu A, Hegg R, Tausch C, Seo JH, Tsai YF, Ratnayake J, McNally V, Ross G, Cortes J (2013) Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA). Ann Oncol 24(9):2278–2284. https://doi.org/10.1093/annonc/mdt182
Swain SM, Ewer MS, Viale G, Delaloge S, Ferrero JM, Verrill M, Colomer R, Vieira C, Werner TL, Douthwaite H, Bradley D, Waldron-Lynch M, Kiermaier A, Eng-Wong J, Dang C (2018) Pertuzumab, trastuzumab, and standard anthracycline- and taxane-based chemotherapy for the neoadjuvant treatment of patients with HER2-positive localized breast cancer (BERENICE): a phase II, open-label, multicenter, multinational cardiac safety study. Ann Oncol 29(3):646–653. https://doi.org/10.1093/annonc/mdx773
Choi JDW, Hughes TMD, Marx G, Rutovitz J, Hasovits C, Ngui NK (2019) Pathological outcomes of HER2-positive non-metastatic breast cancer patients treated with neoadjuvant dual anti-HER2 therapy and taxane: an Australian experience. Asia-Pac J Clin Oncol. https://doi.org/10.1111/ajco.13178
Fasching PA, Hartkopf AD, Gass P, Haberle L, Akpolat-Basci L, Hein A, Volz B, Taran FA, Nabieva N, Pott B, Overkamp F, Einarson H, Hadji P, Tesch H, Ettl J, Luftner D, Wallwiener M, Muller V, Janni W, Fehm TN, Schneeweiss A, Untch M, Pott D, Lux MP, Geyer T, Liedtke C, Seeger H, Wetzig S, Hartmann A, Schulz-Wendtland R, Belleville E, Wallwiener D, Beckmann MW, Brucker SY, Kolberg HC (2019) Efficacy of neoadjuvant pertuzumab in addition to chemotherapy and trastuzumab in routine clinical treatment of patients with primary breast cancer: a multicentric analysis. Breast Cancer Res Treat 173(2):319–328. https://doi.org/10.1007/s10549-018-5008-3
Ogston KN, Miller ID, Payne S, Hutcheon AW, Sarkar TK, Smith I, Schofield A, Heys SD (2003) A new histological grading system to assess response of breast cancers to primary chemotherapy: prognostic significance and survival. Breast 12(5):320–327. https://doi.org/10.1016/s0960-9776(03)00106-1
Symmans WF, Peintinger F, Hatzis C, Rajan R, Kuerer H, Valero V, Assad L, Poniecka A, Hennessy B, Green M, Buzdar AU, Singletary SE, Hortobagyi GN, Pusztai L (2007) Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol 25(28):4414–4422. https://doi.org/10.1200/JCO.2007.10.6823
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45(2):228–247. https://doi.org/10.1016/j.ejca.2008.10.026
Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thurlimann B, Senn HJ (2013) Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol 24(9):2206–2223. https://doi.org/10.1093/annonc/mdt303
Untch M, Jackisch C, Schneeweiss A, Conrad B, Aktas B, Denkert C, Eidtmann H, Wiebringhaus H, Kummel S, Hilfrich J, Warm M, Paepke S, Just M, Hanusch C, Hackmann J, Blohmer JU, Clemens M, Darb-Esfahani S, Schmitt WD, Dan Costa S, Gerber B, Engels K, Nekljudova V, Loibl S, von Minckwitz G (2016) Nab-paclitaxel versus solvent-based paclitaxel in neoadjuvant chemotherapy for early breast cancer (GeparSepto-GBG 69): a randomised, phase 3 trial. Lancet Oncol 17(3):345–356. https://doi.org/10.1016/S1470-2045(15)00542-2
van Ramshorst MS, van der Voort A, van Werkhoven ED, Mandjes IA, Kemper I, Dezentje VO, Oving IM, Honkoop AH, Tick LW, van de Wouw AJ, Mandigers CM, van Warmerdam LJ, Wesseling J, Vrancken Peeters MT, Linn SC, Sonke GS (2018) Neoadjuvant chemotherapy with or without anthracyclines in the presence of dual HER2 blockade for HER2-positive breast cancer (TRAIN-2): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 19(12):1630–1640. https://doi.org/10.1016/S1470-2045(18)30570-9
Yoon GY, Chae EY, Cha JH, Shin HJ, Choi WJ, Kim HH, Kim JE, Kim SB (2019) Imaging and clinicopathologic features associated with pathologic complete response in HER2-positive breast cancer receiving neoadjuvant chemotherapy with dual HER2 blockade. Clin Breast Cancer. https://doi.org/10.1016/j.clbc.2019.06.015
Singh JC, Mamtani A, Barrio A, Morrow M, Sugarman S, Jones LW, Yu AF, Argolo D, Smyth LM, Modi S, Schweber S, Boafo C, Patil S, Norton L, Baselga J, Hudis CA, Dang C (2017) Pathologic complete response with neoadjuvant doxorubicin and cyclophosphamide followed by paclitaxel with trastuzumab and pertuzumab in patients with HER2-positive early stage breast cancer: a single center experience. Oncologist 22(2):139–143. https://doi.org/10.1634/theoncologist.2016-0268
Foldi J, Mougalian S, Silber A, Lannin D, Killelea B, Chagpar A, Horowitz N, Frederick C, Rispoli L, Burrello T, Abu-Khalaf M, Sabbath K, Sanft T, Brandt DS, Hofstatter EW, Hatzis C, DiGiovanna MP, Pusztai L (2018) Single-arm, neoadjuvant, phase II trial of pertuzumab and trastuzumab administered concomitantly with weekly paclitaxel followed by 5-fluoruracil, epirubicin, and cyclophosphamide (FEC) for stage I-III HER2-positive breast cancer. Breast Cancer Res Treat 169(2):333–340. https://doi.org/10.1007/s10549-017-4653-2
van Ramshorst MS, Loo CE, Groen EJ, Winter-Warnars GH, Wesseling J, van Duijnhoven F, Peeters MTV, Sonke GS (2017) MRI predicts pathologic complete response in HER2-positive breast cancer after neoadjuvant chemotherapy. Breast Cancer Res Treat 164(1):99–106. https://doi.org/10.1007/s10549-017-4254-0
De Los Santos JF, Cantor A, Amos KD, Forero A, Golshan M, Horton JK, Hudis CA, Hylton NM, McGuire K, Meric-Bernstam F, Meszoely IM, Nanda R, Hwang ES (2013) Magnetic resonance imaging as a predictor of pathologic response in patients treated with neoadjuvant systemic treatment for operable breast cancer. Translational Breast Cancer Research Consortium trial 017. Cancer 119(10):1776–1783. https://doi.org/10.1002/cncr.27995
Gampenrieder SP, Peer A, Weismann C, Meissnitzer M, Rinnerthaler G, Webhofer J, Westphal T, Riedmann M, Meissnitzer T, Egger H, Klaassen Federspiel F, Reitsamer R, Hauser-Kronberger C, Stering K, Hergan K, Mlineritsch B, Greil R (2019) Radiologic complete response (rCR) in contrast-enhanced magnetic resonance imaging (CE-MRI) after neoadjuvant chemotherapy for early breast cancer predicts recurrence-free survival but not pathologic complete response (pCR). Breast Cancer Res: BCR 21(1):19. https://doi.org/10.1186/s13058-018-1091-y
Weber JJ, Jochelson MS, Eaton A, Zabor EC, Barrio AV, Gemignani ML, Pilewskie M, Van Zee KJ, Morrow M, El-Tamer M (2017) MRI and prediction of pathologic complete response in the breast and axilla after neoadjuvant chemotherapy for breast cancer. J Am Coll Surg 225(6):740–746. https://doi.org/10.1016/j.jamcollsurg.2017.08.027
Marty M, Cognetti F, Maraninchi D, Snyder R, Mauriac L, Tubiana-Hulin M, Chan S, Grimes D, Anton A, Lluch A, Kennedy J, O'Byrne K, Conte P, Green M, Ward C, Mayne K, Extra JM (2005) Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: the M77001 study group. J Clin Oncol 23(19):4265–4274. https://doi.org/10.1200/JCO.2005.04.173
Acknowledgements
The authors would like to thank the NEOPETRA study investigators for their contribution to the study: Fernando Moreno Antón, Hospital Clínico San Carlos, Madrid (Spain); Salvador Blanch Tormo, Instituto Valenciano de Oncología, Valencia (Spain); Noelia Martínez Jañez, Hospital Ramón y Cajal, Madrid (Spain); Elena Galve Calvo, Hospital de Basurto, Bilbao (Spain), Jesús García Mata, Complejo Hospitalario de Orense, Orense (Spain); and Laura García, HM Sanchinarro, Madrid (Spain). We also thank the patients who consented to participate in this study. We thank Roche Farma S.A. for supporting the study. In particular, we would like to acknowledge Pablo López Pedrola and Marian Terrancle from Roche Farma for their support during the study. The authors would also like to thank Cristina Vidal and Antonio Torres, from Dynamic Science (Spain), for their medical writing and editorial support, funded by Roche Farma S.A.
Funding
The study was sponsored by Roche Farma S.A.
Author information
Authors and Affiliations
Contributions
SGS and JIC have contributed to the design, acquisition of data, data analysis and interpretation, and manuscript preparation and review. CS, EC, JLA, PM, MSE, IG, AL, ED, SS, and MRB have contributed to the acquisition of data and manuscript review. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
S. González-Santiago has received speaker honoraria from Amgen, Eisai, Novartis, Roche, Pfizer and AstraZeneca, has served in an advisory role for Amgen, Novartis, Roche, Celgene, AstraZeneca and Pfizer, and has received travel expenses from Pfizer, MSD, and Roche. E. Ciruelos has received personal fees from Roche, Lilly, Novartis, and Pfizer. S. Servitja reported speaker bureau honoraria and travel support from Roche. M. Ruiz Borrego has received a speaker’s grant and has served on the advisory board for Roche. The other authors declare that they do not have any conflict of interest.
Consent to publish
Not applicable as this manuscript does not contain any individual person’s data.
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki and its later amendments. The Research Ethics Committee of Parc Taulí Hospital (Sabadell, Barcelona Spain) approved this study.
Informed consent
Written informed consent was obtained from all individual participants included in the study to collect data from medical charts retrospectively.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary file1 (EPS 2263 kb)
Supplementary Figure 1. pCR rates with neoadjuvant dual HER2 blockade with pertuzumab and trastuzumab in the neoadjuvant trials in patients with HER2-positive BC. A: Anthracyclines; T: Taxanes; TP: Trastuzumab+pertuzumab; Pbc: Platinum based combinations; A→T+TP; Anthracyclines followed by taxanes and trastuzumab+pertuzumab; A + TP → T + Pbc + TP; Anthracyclines plus trastuzumab + pertuzumab followed by taxanes, platinum-based chemotherapy and trastuzumab + pertuzumab; A + T + TP: Anthracyclines + taxanes + trastuzumab + pertuzumab given concurrently; T + TP → A + C +TP; Taxanes plus trastuzumab + pertuzumab followed by anthracyclines, cyclophosphamide and trastuzumab + pertuzumab; T + Pbc + TP: Taxanes, platinum-based chemotherapy, and trastuzumab + pertuzumab; T + TP: taxanes plus trastuzumab + pertuzumab; pCR: Pathological complete response.
Rights and permissions
About this article
Cite this article
González-Santiago, S., Saura, C., Ciruelos, E. et al. Real-world effectiveness of dual HER2 blockade with pertuzumab and trastuzumab for neoadjuvant treatment of HER2-positive early breast cancer (The NEOPETRA Study). Breast Cancer Res Treat 184, 469–479 (2020). https://doi.org/10.1007/s10549-020-05866-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-020-05866-1