Abstract
Purpose
To explore the optimal type of breast reconstruction and the time interval to postmastectomy radiotherapy (PMRT) associated with lower complications in breast cancer patients receiving neoadjuvant chemotherapy.
Methods
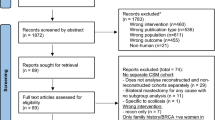
We reviewed the medical records of 300 patients who received neoadjuvant chemotherapy, mastectomy with breast reconstruction and PMRT at our institution from 2000 to 2017. Reconstruction types included autologous flaps (AR), single-stage-direct-to-implant and two-stages expander/implant (TE/I). The primary endpoint was the rate of reconstruction complications including infection, skin and fat necrosis. Subgroup analysis compared rates of capsular contracture, implant rupture, implant exposure and overall implant failure in single-stage-direct-to-implant to TE/I. The secondary endpoint was identifying the time interval between surgery with immediate implant-based reconstruction and PMRT associated with lower probability of implant failure. Logistic regression models, Kaplan–Meier estimates and Polynomial regression were used to assess endpoints.
Results
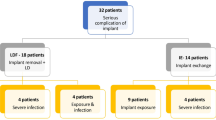
The median follow-up was 43.5 months. 29.3%, 28.3% and 42.4% of the cohort had AR, TE/I and single-stage-direct-to-implant D, respectively. The 5-year cumulative incidence rate of complications was 14.0%, 29.7% and 19.4% for AR, TE/I and single-stage-direct-to-implant, respectively (Log rank p = 0.02). Multivariate analysis showed significant association between TE/I and higher risk of infection (OR 8.1, p = 0.009) compared to AR, while single-stage-direct-to-implant and AR were comparable (OR 3.2, p = 0.2). On subgroup analysis, TE/I was significantly associated with higher rates of implant failure. The mean wait time to deliver PMRT after immediate reconstruction with no adjuvant chemotherapy was 8.4 and 10.7 weeks in single-stage-direct-to-implant and TE/I, respectively (p < 0.005). Delivering PMRT after 8 weeks of surgery yielded 10% probability of reconstruction failure in single-stage-direct-to-implant versus 40% in TE/I.
Conclusion
In comparison to two stages reconstruction, single-stage-direct-to-implant following neoadjuvant chemotherapy has lower complications and offers timely delivery of PMRT.



Similar content being viewed by others
References
Pollom EL, Qian Y, Chin AL et al (2018) Rising rates of bilateral mastectomy with reconstruction following neoadjuvant chemotherapy. Int J Cancer 143(12):3262–3272
Gusic LH, Walsh K, Flippo-Morton T, Sarantou T, Boselli D, White RL Jr (2018) Rationale for mastectomy after neoadjuvant chemotherapy. Am Surg 84(1):126–132
Agarwal S, Kidwell KM, Farberg A, Kozlow JH, Chung KC, Momoh AO (2015) Immediate reconstruction of the radiated breast: recent trends contrary to traditional standards. Ann Surg Oncol 22(8):2551–2559
Ho AY, Hu ZI, Mehrara BJ, Wilkins EG (2017) Radiotherapy in the setting of breast reconstruction: types, techniques, and timing. Lancet Oncol 18(12):e742–e753
NATIONAL COMPREHENSIVE CANCER NETWORK® N, NCCN Guidelines®, .
Oliver JD, Boczar D, Huayllani MT et al (2019) Postmastectomy radiation therapy (PMRT) before and after 2-stage expander-implant breast reconstruction: a systematic review. Medicina (Kaunas, Lithuania). 55(6):226
Frasier LL, Holden S, Holden T et al (2016) Temporal trends in postmastectomy radiation therapy and breast reconstruction associated with changes in national comprehensive cancer network guidelines trends in postmastectomy radiation therapy and breast reconstructiontrends in postmastectomy radiation therapy and breast reconstruction. JAMA Oncol 2(1):95–101
Colwell AS (2015) Current strategies with 1-stage prosthetic breast reconstruction. Gland Surg 4(2):111–115
Colwell AS (2012) Direct-to-implant breast reconstruction. Gland Surg 1(3):139–141
Naoum GE, Salama L, Ho A et al (2019) The impact of chest wall boost on reconstruction complications and local control in patients treated for breast cancer. Int J Radiat Oncol Biol Phys 105(1):155–164
Goodwin SJ, McCarthy CM, Pusic AL et al (2005) Complications in smokers after postmastectomy tissue expander/implant breast reconstruction. Ann Plast Surg 55(1):16–19 (discussion 19–20)
Garvey PB, Villa MT, Rozanski AT, Liu J, Robb GL, Beahm EK (2012) The advantages of free abdominal-based flaps over implants for breast reconstruction in obese patients. Plast Reconstr Surg 130(5):991–1000
Momoh AO, Griffith KA, Hawley ST et al (2020) Postmastectomy breast reconstruction: exploring plastic surgeon practice patterns and perspectives. Plast Reconstr Surg 145(4):865–876
Frey JD, Choi M, Karp NS (2017) The effect of neoadjuvant chemotherapy compared to adjuvant chemotherapy in healing after nipple-sparing mastectomy. Plast Reconstr Surg 139(1):10e–19e
Oh E, Chim H, Soltanian HT (2012) The effects of neoadjuvant and adjuvant chemotherapy on the surgical outcomes of breast reconstruction. J Plast Reconstr Aesthet Surg JPRAS 65(10):e267–280
Song J, Zhang X, Liu Q et al (2014) Impact of neoadjuvant chemotherapy on immediate breast reconstruction: a meta-analysis. PLoS ONE 9(5):e98225
Ilonzo N, Tsang A, Tsantes S, Estabrook A, Thu Ma AM (2017) Breast reconstruction after mastectomy: a ten-year analysis of trends and immediate postoperative outcomes. Breast (Edinburgh, Scotland) 32:7–12
Dolen UC, Schmidt AC, Um GT et al (2016) Impact of neoadjuvant and adjuvant chemotherapy on immediate tissue expander breast reconstruction. Ann Surg Oncol 23(7):2357–2366
Hu YY, Weeks CM, In H et al (2011) Impact of neoadjuvant chemotherapy on breast reconstruction. Cancer 117(13):2833–2841
Smith BL, Tang R, Rai U et al (2017) Oncologic safety of nipple-sparing mastectomy in women with breast cancer. J Am Coll Surg 225(3):361–365
Smith BL, Coopey SB (2018) Nipple-sparing mastectomy. Adv Surg 52(1):113–126
Dull B, Conant L, Myckatyn T, Tenenbaum M, Cyr A, Margenthaler JA (2017) Nipple-sparing mastectomies: clinical outcomes from a single academic institution. Mol Clin Oncol 6(5):737–742
Margulies IG, Salzberg CA (2019) The use of acellular dermal matrix in breast reconstruction: evolution of techniques over 2 decades. Gland Surg 8(1):3–10
Sbitany H, Langstein HN (2011) Acellular dermal matrix in primary breast reconstruction. Aesthet Surg J 31(7 Suppl):30s–37s
Jagsi R, Momoh AO, Qi J et al (2018) Impact of radiotherapy on complications and patient-reported outcomes after breast reconstruction. J Natl Cancer Inst 110(2):157
Riggio E, Toffoli E, Tartaglione C, Marano G, Biganzoli E (2019) Local safety of immediate reconstruction during primary treatment of breast cancer. Direct-to-implant versus expander-based surgery. J Plast Reconstr Aesthet Surg JPRAS. 72(2):232–242
Dikmans REG, Negenborn VL, Bouman M-B et al (2017) Two-stage implant-based breast reconstruction compared with immediate one-stage implant-based breast reconstruction augmented with an acellular dermal matrix: an open-label, phase 4, multicentre, randomised, controlled trial. Lancet Oncol 18(2):251–258
Potter S, Wilson RL, Harvey J, Holcombe C, Kirwan CC (2017) Results from the BRIOS randomised trial. Lancet Oncol 18(4):e189
Naoum GE, Salama L, Niemierko A et al (2019) Single stage direct-to-implant breast reconstruction has less complication rates than tissue expander/implant and comparable rates to autologous reconstruction in patients receiving postmastectomy radiation. Int J Radiat Oncol Biol Phys 106:514
Yoon J, Xie Y, Heins D, Zhang R (2018) Modeling of the metallic port in breast tissue expanders for photon radiotherapy. J Appl Clin Med Phys 19(3):205–214
Damast S, Beal K, Ballangrud A et al (2006) Do metallic ports in tissue expanders affect postmastectomy radiation delivery? Int J Radiat Oncol Biol Phys 66(1):305–310
Haubner F, Ohmann E, Pohl F, Strutz J, Gassner HG (2012) Wound healing after radiation therapy: review of the literature. Radiat Oncol 7(1):162
Funding
N/A, no funding available for this study.
Author information
Authors and Affiliations
Contributions
Andrzej Niemierko was responsible for statistical analysis.
Corresponding author
Ethics declarations
Conflict of interest
Alphonse Taghian is on the Scientific Advisory Board of Puretech Health and Consultant in VisionRT. None of it is relevant to the current study. The rest of the authors have nothing to declare.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Institutional IRB approval Protocol #: 2017P001175.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Naoum, G.E., Oladeru, O.T., Niemierko, A. et al. Optimal breast reconstruction type for patients treated with neoadjuvant chemotherapy, mastectomy followed by radiation therapy. Breast Cancer Res Treat 183, 127–136 (2020). https://doi.org/10.1007/s10549-020-05747-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-020-05747-7