Abstract
Purpose
Breast cancer-related lymphedema (BCRL) represents a lifelong risk for breast cancer survivors and once acquired becomes a lifelong burden. This review summarizes current BCRL prevention and treatment strategies.
Findings
Risk factors for BCRL have been extensively studied and their identification has affected breast cancer treatment practice, with sentinel lymph node removal now standard of care for patients with early stage breast cancer without sentinel lymph node metastases. Early surveillance and timely management aim to reduce BCRL incidence and progression, and are further facilitated by patient education, which many breast cancer survivors report not having adequately received. Surgical approaches to BCRL prevention include axillary reverse mapping, lymphatic microsurgical preventative healing (LYMPHA) and Simplified LYMPHA (SLYMPHA). Complete decongestive therapy (CDT) remains the standard of care for patients with BCRL. Among CDT components, facilitating manual lymphatic drainage (MLD) using indocyanine green fluorescence lymphography has been proposed. Intermittent pneumatic compression, nonpneumatic active compression devices, and low-level laser therapy appear promising in lymphedema management. Reconstructive microsurgical techniques such as lymphovenous anastomosis and vascular lymph node transfer are growing surgical considerations for patients as well as liposuction-based procedures for addressing fatty fibrosis formation from chronic lymphedema. Long-term self-management adherence remains problematic, and lack of diagnosis and measurement consensus precludes a comparison of outcomes. Currently, no pharmacological approaches have proven successful.
Conclusion
Progress in prevention and treatment of BCRL continues, requiring advances in early diagnosis, patient education, expert consensus and novel treatments designed for lymphatic rehabilitation following insults.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer-related lymphedema (BCRL) is one of the most feared complications of breast cancer [1, 2]. It represents a lifelong burden for many and a lifelong risk for nearly all breast cancer survivors. It can neither be cured nor easily concealed in advanced stages [3]. Its long-term burden extends beyond considerable symptoms (e.g., arm swelling, pain, limited function necessitating compensatory movement strategies) [4], to significantly impact quality-of-life (QoL), psychosocial interactions, and emotional wellbeing [5, 6], as well as cause substantial financial burdens to patients, caregivers, payers and society [7].
There is no single tool to assess BCRL, but various objective tools and more subjective clinical examination. Lack of standardized methods and protocols for assessing lymphedema has been problematic for decades limiting understanding of BCRL incidence and treatment outcomes. Table 1 shows frequently reported objective BCRL assessment methods. Patient-reported symptoms also have diagnostic value [8]. Clinical assessment methods used are typically institution- and equipment-dependent. Diagnostic threshold values may differ for a given method. BCRL prevalence estimates therefore vary widely. With an estimated 3.8 million breast cancer survivors currently in the U.S. [9], the number of patients affected by BCRL likely approaches one million. As better treatment methods extend survival in breast cancer, BCRL will represent an increasingly important consideration where identifying an accurate and reproducible tool that is readily accessible would have a momentous impact on BCRL management and treatment [10].
BCRL prevention
Risk factors for BCRL have been extensively reviewed [11,12,13,14,15,16,17,18,19,20,21] (Online Resource 1: Supplementary Table 1, Supplementary Table 2). Lymphadenectomy is the primary treatment-related risk factor for BCRL in patients with breast cancer undergoing surgery. A higher number of lymph nodes dissected is associated with increased risk [11], as is axillary (ALND) versus sentinel lymph node dissection (SLND). For example, a large, long-term study of patients with invasive breast cancer reported cumulative BCRL rates of 24.9% and 8% in the axial lymph node alone and sentinel lymph node alone cohorts, respectively [22]. Mastectomy has been associated with a significantly higher BCRL risk than lumpectomy [15], and evidence suggests that immediate post-mastectomy breast reconstruction lowers BCRL risk [16, 23, 24]. Identifying these treatment-related risk factors has affected standard surgical practice, with SLND now the standard of care for women with early stage breast cancer, and ALND contraindicated in those without sentinel lymph node metastasis [25].
Radiotherapy is also associated with increased BCRL risk [26]. A randomized study of women treated with breast-conserving surgery and adjuvant systemic therapy found that patients treated with regional nodal irradiation had a higher rate of lymphedema (8.4% vs. 4.5%; P = 0.001) at 9.5 years than patients not receiving it [27] (see Table 2).
Among proposed non-treatment-related, independent risk factors for BCRL are age [28], body mass index (BMI) at baseline [11, 20, 29], genetic factors [30], post-operative infection [31], race or ethnicity [32], and the presence of subclinical edema [33].
Non-surgical approaches to prevention
Early surveillance with timely intervention reduces both BCRL incidence and severity [34]. A retrospective study of breast cancer survivors compared those who had begun bioimpedance spectroscopy (BIS) monitoring pre-surgery or within 90 days post-surgery with a cohort for which BIS monitoring began later (median 2.1 years) [35]. Significantly more women in the latter group were diagnosed with BCRL (any grade, 39% vs. 14%; P < 0.001) and BCRL severity was also higher (stage II-III, 24% vs. 4%). Prevention benefits may depend on the BCRL assessment method employed, with BIS providing more precise identification of patients more likely to benefit from early compression intervention than tape measurement in one recent study [36]. Serial near-infrared fluorescence lymphatic imaging (NIRF-LI) was associated with 83% and 86% positive and negative BCRL predictivity, respectively [37]. BCRL dermal backflow often appeared months before arm swelling, enabling earlier recognition of lymphatic dysfunction to triage for earlier treatment.
Other studies have also associated early surveillance combined with timely intervention for sub-clinical lymphedema with low rates of progression to clinical BCRL [38,39,40,41,42]. Early interventions include use of compression sleeves and manual lymph drainage (MLD) [43, 44]. In one prospective study of patients undergoing ALND at high risk for BCRL, regular BIS assessments at 3–6 month intervals were followed by short-term physical therapy, compression garments, and lymphedema education for those with sub-clinical lymphedema [38]. At a median 19-month follow-up, the incidence of clinical BCRL was a remarkably low 4.4%. Another study of structured BIS surveillance and early intervention reported a 3% rate of BCRL (median 24-month follow-up) [39]. In a randomized study, prophylactic compression sleeves usage significantly reduced arm swelling incidence (HR 0.61; P = 0.004) relative to the control group as measured by BIS among women undergoing ALND [44]. This growing body of evidence for BCRL surveillance impact underscores the critical need for elevating basic surveillance model requirements across the U.S. for triage to basic early intervention.
Patient education is an important component of BCRL prevention. Given the benefits of early BCRL treatment and in light of evidence that patient-reported arm symptoms (e.g., clothing or jewelry tightness, arm heaviness) may be prodromal [45,46,47], all breast cancer patients should know the importance of contacting their healthcare providers immediately should such symptoms arise [48,49,50]. Since cellulitis may act as a trigger for BCRL, patients should also be cautioned to avoid infections [49]. Although pretreatment lymphedema education is recommended to reduce BCRL incidence [51, 52], many patients report never having received this information [52]. Efforts must be made to ensure individualized, patient-centric education—with touchpoints throughout their cancer care—is being provided and retained by the patient. Of note, an international consensus for preventive intervention for BCRL was recently published, which provides recommendations to assist in clinical guidelines development [53]. The recommendation of high consensus involved the critical importance of adequate patient education about lymphedema, ensuring the patient understands the information and is empowered to take an active approach.
The Prospective Surveillance Model (PSM) is a comprehensive approach to survivorship healthcare for women with breast cancer [54, 55]. It provides time points for assessments and education from diagnosis through long-term survivorship, emphasizing identification and management of impairments (including BCRL) and health-promoting behaviors. An analysis estimated the cost to manage early stage BCRL per patient year using PSM at $636 and the cost to manage late-stage BCRL at $3125 per patient year, making PSM attractive from a health economics standpoint [56]. The feasibility of PSM for BCRL prevention in real-world clinical practice has been demonstrated [57, 58]. BIS monitoring with portable equipment during in-home visits may also be a viable component of the PSM, particularly for patients living far from large treatment centers and/or at high BCRL risk [59].
Surgical approaches to prevention
Axillary reverse mapping (ARM) is a technique for identifying and sparing arm lymphatic drainage in patients undergoing ALND or SLND, aimed to minimize lymphedema risk [60]. Injecting blue or fluorescent dye into the arm allows visual differentiation of arm lymphatics from technetium-labeled breast lymphatics, and consequently their preservation during dissection. In a large, prospective study of ARM, 26-month lymphedema rates (increased water volume displacement ≥ 20%) were only 0.8% and 6.5% for patients who underwent SLND and ALND, respectively [61]. In some cases, however, crossover between the lymphatics from breast and arm has been noted, and metastatic disease may be present in ARM nodes. In addition, not all ARM nodes can be identified [62, 63].
Constructing lymphatic-venous anastomoses (LVAs) is a growing approach to treating secondary lymphedema. In Lymphatic Microsurgical Preventative Healing (LYMPHA), LVAs are used for primary prevention of arm lymphedema at the time of axillary dissection [64]. Using supermicrosurgery, arm lymphatics are connected with a collateral branch of the axillary vein distal to a competent valve [16, 65]. Among 46 women with breast cancer undergoing ALND randomized to no preventative surgical approach or to LYMPHA, lymphedema had occurred in 4.3% and 30.4% of the LYMPHA and control groups, respectively (p < 0.05) at 18-months [66].
While LYMPHA may be a promising technique, expertise in microsurgery, coordination between breast and plastic surgeons, and an upfront decision before surgery are required [67]. Additionally, there is risk associated with LYMPHA and a surgical learning curve. A 2021 study reported that 85% of breast surgeons reported not offering LYMPHA [68]. A simplified version of LYMPHA not requiring microsurgery has been described (SLYMPHA), with the procedure lowering BCRL incidence from 32 to 16% in one study [69]. Further surgical development for addressing lymphatic impairments is warranted along with algorithms for identifying best surgical candidates for the various interventions along with long-term surgical outcomes.
Treatment of BCRL
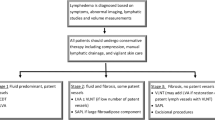
There is currently no approved drug therapy for lymphedema [70]. Recent clinical trials of pharmacological agents for the treatment of BCRL are summarized in Online Resource 1: Supplementary Table 3. Approaches to BCRL management are outlined in Fig. 1 and discussed below.
Complete decongestive therapy (CDT)
The standard of care for patients with BCRL is Complete Decongestive Therapy (CDT, Complex Decongestive Therapy, Combined Physical Therapy [CPT], Complex Decongestive Physiotherapy [CDP], or Complex Lymphoedema Treatment [CLT]) [71, 72]. This multicomponent, multidisciplinary approach consists of an intensive treatment phase followed by a maintenance phase [73, 74].
The treatment phase focuses on MLD, multilayer short-stretch compression bandage (CB) wrapping and/or Velcro or other adjustable wraps, exercise to improve lymph flow [75], and meticulous skin care of the affected area [72]. MLD appears to stimulate lympholymphatic or lymphovenous anastomoses (LVA) [76]. The ability of MLD to facilitate transit of lymphatic fluids has been demonstrated using ICG fluorescence lymphography [77], potentially allowing for personalized MLD treatment [78]. Phase 1 of CDT is performed/supervised by a licensed clinician, typically with a physical or occupational degree, with specialized lymphedema training with a focus on maximal volume reduction and patient training [79]. Subsequently, patients are transitioned into their long-term maintenance phase (Phase 2) which involves self-care management of their chronic lymphedema using day and possibly night time compression, exercise, skin care, home pneumatic compression pumps and self lymphatic massage, to name a few, to maintain their optimal decongested state [79]. Resistance exercise has proven safe and demonstrated arm volume reduction benefits [80, 81]. Lymphedema maintenance being life-long, adherence to this complex, multi-modal regimen becomes problematic for many breast cancer survivors [82, 83].
There is little evidence that MLD alone is effective in BCRL management. A 2010 review of 16 trials found no consensus on the effectiveness of MLD alone [76]. In practice, however, MLD is used primarily as just one component of CDT. In that setting some [84, 85], but not all [86,87,88,89,90] analyses suggest that MLD may contribute to CDT benefit. In a recent systematic review and meta-analysis of 11 randomized controlled trials the addition of MLD to control treatments was associated with significant (P = 0.02) improvements in pain intensity but not arm volume reductions or QoL [90]. In a recent trial randomizing patients to fluorescence-guided MLD, normal MLD, or placebo MLD (all in combination with standardized CDT), all 3 groups had similar improvements in fluid accumulation and skin elasticity [91].
It is important to note that BCRL may affect the upper quadrant and/or the arm, thus therapy (including compression) addresses the area(s) of impairment. During the initial CDT phase, bandaging is applied to the limb and/or upper quadrant immediately after MLD treatment. The multilayer bandage, removed only for washing and MLD, applies a resting pressure during limb relaxation and a working pressure upon muscle contraction, mechanically stimulating the smooth muscle of lymph vessels [92]. Distinct from this, during the life-long maintenance phase of CDT, patients are fitted with compression garments to maintain the volume reduction achieved initially. These are not expected to provide any additional volume reductions yet are necessary for lymphedema containment and need to be properly fitted by a specialist. Compression garments provide transverse and longitudinal stretch with a high-to-low pressure gradient from above the wrist to the upper arm [93]. A full-sleeve compression garment is usually worn, sometimes with a glove to prevent dermal backflow. Compression garments must be replaced frequently, which adds to the financial burden as some insurers (e.g., Medicare) do not cover costs of compression garments (except compression bras), [94] though efforts are underway to improve insurance coverage.
Although CDT is regarded as the cornerstone of BCRL therapy [18, 65], evidence for its effectiveness varies [95]. CDT was found to be effective in reducing lymphedema in a systematic review of lymphedema studies from 2004 to 2011, although levels of evidence were only moderately strong [72, 96]. A 2007 retrospective analysis of 250 breast cancer survivors treated with CDT (55%), MLD (32%) or a home program (13%) agreed that these methods were collectively effective, with a mean 47% lymphedema volume reduction at 1 year (p < 0.0001) [97]. Not all studies, however, support the value of CDT relative to other, less resource-intensive treatments in BCRL. In a small randomized non-inferiority trial, compression bandaging plus exercise provided similar arm volume reductions and QoL improvements as CDT in post-mastectomy patients with arm edema [88].
A 2013 randomized trial compared elastic compression garments consisting of sleeve (30–40 mmHg) and glove alone (the control group) with CDT (intervention group) [98]. Mean excess arm volume reductions were 29.0% and 22.6%, respectively (P = 0.34) and QoL was similar in both groups. The trial was unable to demonstrate a significant improvement with CDT relative to compression garments, a surprising result given that compression garments are intended for containment and are not designed to enhance lymphatic pumping [10] warranting further investigation on this more simplified intervention and ideal patient candidate algorithm.
CDT is contraindicated in several conditions. Relative contraindications include uncontrolled hypertension, paralysis, diabetes, and bronchial asthma, while absolute contraindications include acute infections, uncontrolled congestive heart failure, and deep vein thrombosis [92]. Although it has been postulated that CDT/MLD might mobilize dormant tumor cells, thereby promoting cancer metastasis [92], studies suggest that this is not the case and CDT should not be withheld from patients with metastatic cancer [99].
Another compression method, intermittent pneumatic compression (IPC) as an adjunct to CDT was associated with additional mean volume reductions when used in either the initial treatment or the maintenance phase [100]. A 2022 systematic review concluded that based on existing evidence, IPC may provide an acceptable home-based treatment modality in addition to wearing compression garments in select patients with lymphedema [101]. Finally, a novel nonpneumatic active compression device (NPCD) that does not require patients to be immobile during use was recently evaluated in a randomized crossover trial [102]. Although results were encouraging, as with IPC, further studies are needed.
Photobiomodulation (PBM)
Photobiomodulation (PBM), also known as low-level laser therapy (LLLT), is a type of phototherapy that uses light of wavelengths between 650 and 1000 nm delivered at low irradiance to the target site [103]. PBM has been shown to reduce inflammation, promote lymphatic mobility and regeneration, and prevent/manage fibrosis [103, 104]. Studies have examined PBM outcomes including arm volume/circumference, symptoms, and QoL [105,106,107,108,109]. Systematic reviews and meta-analyses have differed in their conclusions regarding its effectiveness in patients with BCRL [103, 110,111,112]. Larger randomized trials employing standardized protocols for treatment and assessment may clarify its potential benefit in this patient population, particularly when used in combination with CDT.
Surgical treatment of BCRL
Although not necessarily with curative potential, advances in surgical approaches to BCRL treatment are likely to modify the current practice of typically reserving them for patients with lymphedema refractory to more conservative methods. Ultimately, the surgeries may go hand in hand with other conservative approaches. Lymphedema surgeries aim to either restore physiological lymphatic drainage ("reconstructive") or directly remove excess mass ("reductive").
Reconstructive surgeries
LVA is a method of diverting lymph into the venous system, bypassing proximal obstruction. Lymphatic channels are identified, typically using ICG fluorescence imaging, a suitable recipient vein is also identified, and supermicrosurgical techniques are used to create an anastomosis between the two [113]. In patients with BCRL, studies have associated LVA with symptom improvement, arm volume reduction, and, notably, fewer episodes of cellulitis [113, 114]. After recovery, patients are urged to continue their previous therapies and wear compression garments [114]. More recently, however, a study of LVA side-to-end anastomoses in early grade lymphedema reported that it eliminated the need for compression garments later [115]. LVA is not curative, requires a multidisciplinary approach to integrate operative and post-operative management, and is technically demanding, requiring ICG fluorescence, supermicrosurgery instruments, and surgeons proficient in this specialized technique.
Rather than bypass obstructions in existing lymph node drainage, in vascular lymph node transfer (VLNT) an autologous lymph node flap microsurgically harvested from a distant donor site is transplanted to the target area with its blood supply preserved by anastomosing artery and vein in the graft to vessels at the receptor site, which may be axilla, elbow, or wrist. [116]. Donor sites include jejunal mesenteric, groin, lateral thoracic, omental, and submental [117]. Although the mechanism(s) by which lymphatic flow is restored is incompletely understood [118], improved lymphatic transport that results has been demonstrated in numerous studies [65].
A recent study examined outcomes for patients with Stage 2 primary or secondary lymphedema treated with pre-operative conservative therapy followed by VLNT [117]. Two years after surgery significant reductions in limb volume (mean 45.7%; P = 0.002), BIS scores (mean 59.8%; P < 0.001), and cellulitis episodes (97.9%; P < 0.001) were observed, and patient QoL per Lymphedema Life Impact Scale score was improved (mean 61.6%; P < 0.001). Complication rates were low.
Systematic reviews and meta-analyses have supported the benefits of VLNT in patients with lymphedema [119, 120]. In one such study, among patients who underwent lymphoscintigraphy or lymphangiography, 60% demonstrated moderate or significant flow improvement, and 93% reported a high satisfaction level (Fig. 2) [119]. Reports have suggested that VLNT may allow some patients to later reduce or eliminate conservative measures such as compression garment usage [120, 121], thereby ameliorating an economic burden and source of diminished QoL [122, 123].
Reported outcomes in patients with BCRL after VLNT surgery. From a systematic review by Ozturk et al. [119]
Disadvantages of VLNT include risk of iatrogenic lymphedema at the donor site [124]. In a report of patients with secondary upper limb lymphedema undergoing VLNT at a specialized lymphology center, complication rates were 14.3% for irreversible lower limb lymphedema, 21.4% for lymphocele, and 14.3% for donor site pain [124]. Considering that patients likely undergo VLNT in the hope of curing their lymphedema, improvements in surgical techniques and patient identification will be essential for optimizing outcomes. Notably, VLNT changes to lymphatic function are gradual, with months or years needed to achieve full benefit [65], highlighting the importance of patient education in surgical expectations. Further advances in surgical technique(s), timing of surgical intervention (i.e. preventative or management of lymphatic impairment), and improved patient identification will be important in making treatment decisions regarding the role of reconstructive surgeries in BCRL.
Reductive surgeries
In chronic, advanced stage lymphedema a high content of adipose and fibrotic tissues are present [125, 126]. Liposuction-based procedures such as suction assisted protein lipectomy (SAPL) can be used to remove excess solid volume in a lymphedema-affected arm [118, 127]. These techniques are generally reserved for patients with chronic, non-pitting BCRL [125] and do not restore lymphatic function, but reduce limb size for physical functioning improvement, easier daily self-management and more optimal quality of life. Edema volume reductions are rapid, with few complications reported, although it essential to underscore these surgical outcomes are from surgeons highly experienced in this specialized technique [118]. Mean percentage reductions in arm volume of 101–118% at 1–3 years after surgery are typically reported [128,129,130], and are long-lasting [129]. However, the underlying lymphatic impairment is not cured, and the involved region be maintained by constant, life-long compression garment usage [125]. A decreased incidence of infections and improved QoL have been associated with liposuction/SAPL in patients with BCRL [131, 132].
Conclusions
BCRL affects more than one million breast cancer survivors worldwide. Breast cancer survivors face a lifelong risk of BCRL occurrence. It is generally incurable, negatively affects QoL, physical function, and daily activities, and requires lifelong management [56, 92, 133]. Its continuing burden (e.g., wearing expensive compression garments, avoidance of cuts and scratches) ultimately makes for low adherence, enabling more rapid progression and further disability. BCRL screening and education in at-risk patients are imperative, and an individualized approach to goal setting is recommended to improve adherence. Many patients with breast cancer report never having received information about BCRL, however [52], an unacceptable situation that needs to be remedied. Methods such as ARM and LYMPHA at the time of surgery have been shown to be effective in reducing BCRL incidence but come with their own risks and are not always feasible. Newer surgical methods for immediate lymphatic reconstruction will likely play an increasingly important role in BCRL prevention [69, 134, 135].
For breast cancer survivors with BCRL, CDT remains the current standard of care [71, 72]. It comes with high financial, time, and adherence requirements, and is not curative. Novel techniques such as IPC, PBM, and NPCD may have a place in BCRL treatment, but further studies are needed.
Surgical approaches to BCRL treatment continue to emerge with the intention of restoring normal lymphatic flow in patients with lymphedema. LVA and VLNT are two types of reconstructive surgery. Although often effective, they require specialized microsurgical or supermicrosurgical expertise and neither is curative. As these techniques continue to evolve, they may increasingly be used at an earlier stage in selected patients. Reductive surgery via liposuction/SAPL, performed by surgeons experienced in this specialized technique results in immediate volume reduction, but maintaining the new equilibrium requires the constant use of compression garments. Currently no drug has proven safe and effective in treating BCRL.
Assessing the impact of BCRL prophylaxis or treatment requires a comprehensive evaluation of patient- and clinician-reported outcomes. Perhaps the greatest barrier to progress in the prevention and treatment of BCRL is the current lack of standardized measures by which these outcomes can be compared. Progress in optimizing BCRL care must therefore encompass advances in patient education and investigator consensus as well as clinical techniques.
Data availability
Not applicable.
Code availability
Not applicable.
References
Ostby PL, Armer JM, Smith K, Stewart BR (2018) Patient perceptions of barriers to self-management of breast cancer-related lymphedema. West J Nurs Res 40:1800–1817. https://doi.org/10.1177/0193945917744351
Sun F, Hall A, Tighe MP, Brunelle CL, Sayegh HE, Gillespie TC, Daniell KM, Taghian AG (2018) Perometry versus simulated circumferential tape measurement for the detection of breast cancer-related lymphedema. Breast Cancer Res Treat 172:83–91. https://doi.org/10.1007/s10549-018-4902-z
Ridner SH, Bonner CM, Deng J, Sinclair VG (2012) Voices from the shadows: living with lymphedema. Cancer Nurs 35:E18-26. https://doi.org/10.1097/NCC.0b013e31821404c0
Fu MR, Axelrod D, Cleland CM, Qiu Z, Guth AA, Kleinman R, Scagliola J, Haber J (2015) Symptom report in detecting breast cancer-related lymphedema. Breast Cancer (Dove Med Press) 7:345–352. https://doi.org/10.2147/BCTT.S87854
Ahmed RL, Prizment A, Lazovich D, Schmitz KH, Folsom AR (2008) Lymphedema and quality of life in breast cancer survivors: the Iowa Women’s Health Study. J Clin Oncol 26:5689–5696. https://doi.org/10.1200/JCO.2008.16.4731
Fu MR, Kang Y (2013) Psychosocial impact of living with cancer-related lymphedema. Semin Oncol Nurs 29:50–60. https://doi.org/10.1016/j.soncn.2012.11.007
De Vrieze T, Nevelsteen I, Thomis S, De Groef A, Tjalma WAA, Gebruers N, Devoogdt N (2020) What are the economic burden and costs associated with the treatment of breast cancer-related lymphoedema? A systematic review. Support Care Cancer 28:439–449. https://doi.org/10.1007/s00520-019-05101-8
Ridner SH, Shah C, Boyages J, Koelmeyer L, Ajkay N, DeSnyder SM, McLaughlin SA, Dietrich MS (2020) L-Dex, arm volume, and symptom trajectories 24 months after breast cancer surgery. Cancer Med 9:5164–5173. https://doi.org/10.1002/cam4.3188
American Cancer Society (2022) Key statistics for breast cancer. https://www.cancer.org/cancer/breast-cancer/about/how-common-is-breast-cancer.html. Accessed 16 Jan 2023
Shaitelman SF, Cromwell KD, Rasmussen JC, Stout NL, Armer JM, Lasinski BB, Cormier JN (2015) Recent progress in the treatment and prevention of cancer-related lymphedema. CA Cancer J Clin 65:55–81. https://doi.org/10.3322/caac.21253
DiSipio T, Rye S, Newman B, Hayes S (2013) Incidence of unilateral arm lymphoedema after breast cancer: a systematic review and meta-analysis. Lancet Oncol 14:500–515. https://doi.org/10.1016/S1470-2045(13)70076-7
Kilbreath SL, Refshauge KM, Beith JM, Ward LC, Ung OA, Dylke ES, French JR, Yee J, Koelmeyer L, Gaitatzis K (2016) Risk factors for lymphoedema in women with breast cancer: a large prospective cohort. Breast 28:29–36. https://doi.org/10.1016/j.breast.2016.04.011
Penn IW, Chang YC, Chuang E, Chen CM, Chung CF, Kuo CY, Chuang TY (2019) Risk factors and prediction model for persistent breast-cancer-related lymphedema: a 5-year cohort study. Support Care Cancer 27:991–1000. https://doi.org/10.1007/s00520-018-4388-6
Norman SA, Localio AR, Kallan MJ, Weber AL, Torpey HA, Potashnik SL, Miller LT, Fox KR, DeMichele A, Solin LJ (2010) Risk factors for lymphedema after breast cancer treatment. Cancer Epidemiol Biomark Prev 19:2734–2746. https://doi.org/10.1158/1055-9965.EPI-09-1245
Tsai RJ, Dennis LK, Lynch CF, Snetselaar LG, Zamba GK, Scott-Conner C (2009) The risk of developing arm lymphedema among breast cancer survivors: a meta-analysis of treatment factors. Ann Surg Oncol 16:1959–1972. https://doi.org/10.1245/s10434-009-0452-2
Gillespie TC, Sayegh HE, Brunelle CL, Daniell KM, Taghian AG (2018) Breast cancer-related lymphedema: risk factors, precautionary measures, and treatments. Gland Surg 7:379–403. https://doi.org/10.21037/gs.2017.11.04
Kwan ML, Darbinian J, Schmitz KH, Citron R, Partee P, Kutner SE, Kushi LH (2010) Risk factors for lymphedema in a prospective breast cancer survivorship study: the Pathways Study. Arch Surg (Chicago, Ill.: 1950) 145:1055–1063. https://doi.org/10.1001/archsurg.2010.231
Armer JM, Hulett JM, Bernas M, Ostby P, Stewart BR, Cormier JN (2013) Best practice guidelines in assessment, risk reduction, management, and surveillance for post-breast cancer lymphedema. Curr Breast Cancer Rep 5:134–144. https://doi.org/10.1007/s12609-013-0105-0
Kwan JYY, Famiyeh P, Su J, Xu W, Kwan BYM, Jones JM, Chang E, Yip KW, Liu FF (2020) Development and validation of a risk model for breast cancer-related lymphedema. JAMA Netw Open 3:e2024373. https://doi.org/10.1001/jamanetworkopen.2020.24373
Koelmeyer LA, Gaitatzis K, Dietrich MS, Shah CS, Boyages J, McLaughlin SA, Taback B, Stolldorf DP, Elder E, Hughes TM, French JR, Ngui N, Hsu JM, Moore A, Ridner SH (2022) Risk factors for breast cancer-related lymphedema in patients undergoing 3 years of prospective surveillance with intervention. Cancer 128:3408–3415. https://doi.org/10.1002/cncr.34377
McLaughlin SA, Brunelle CL, Taghian A (2020) Breast cancer-related lymphedema: risk factors, ccreening, management, and the impact of locoregional treatment. J Clin Oncol 38:2341–2350. https://doi.org/10.1200/JCO.19.02896
Naoum GE, Roberts S, Brunelle CL, Shui AM, Salama L, Daniell K, Gillespie T, Bucci L, Smith BL, Ho AY, Taghian AG (2020) Quantifying the impact of axillary surgery and nodal irradiation on breast cancer-related lymphedema and local tumor control: long-term results from a prospective screening trial. J Clin Oncol 38:3430–3438. https://doi.org/10.1200/JCO.20.00459
Card A, Crosby MA, Liu J, Lindstrom WA, Lucci A, Chang DW (2012) Reduced incidence of breast cancer-related lymphedema following mastectomy and breast reconstruction versus mastectomy alone. Plast Reconstr Surg 130:1169–1178. https://doi.org/10.1097/PRS.0b013e31826d0faa
Miller CL, Colwell AS, Horick N, Skolny MN, Jammallo LS, O’Toole JA, Shenouda MN, Sadek BT, Swaroop MN, Ferguson CM, Smith BL, Specht MC, Taghian AG (2016) Immediate implant reconstruction is associated with a reduced risk of lymphedema compared to mastectomy alone: a prospective cohort study. Ann Surg 263:399–405. https://doi.org/10.1097/SLA.0000000000001128
Lyman GH, Somerfield MR, Bosserman LD, Perkins CL, Weaver DL, Giuliano AE (2017) Sentinel lymph node biopsy for patients with early-stage breast cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol 35:561–564. https://doi.org/10.1200/JCO.2016.71.0947
Erickson VS, Pearson ML, Ganz PA, Adams J, Kahn KL (2001) Arm edema in breast cancer patients. J Natl Cancer Inst 93:96–111. https://doi.org/10.1093/jnci/93.2.96
Whelan TJ, Olivotto IA, Parulekar WR, Ackerman I, Chua BH, Nabid A, Vallis KA, White JR, Rousseau P, Fortin A, Pierce LJ, Manchul L, Chafe S, Nolan MC, Craighead P, Bowen J, McCready DR, Pritchard KI, Gelmon K, Murray Y, Chapman JA, Chen BE, Levine MN, Investigators MAS (2015) Regional nodal irradiation in early-stage breast cancer. N Engl J Med 373:307–316. https://doi.org/10.1056/NEJMoa1415340
Bevilacqua JL, Kattan MW, Changhong Y, Koifman S, Mattos IE, Koifman RJ, Bergmann A (2012) Nomograms for predicting the risk of arm lymphedema after axillary dissection in breast cancer. Ann Surg Oncol 19:2580–2589. https://doi.org/10.1245/s10434-012-2290-x
McDuff SGR, Mina AI, Brunelle CL, Salama L, Warren LEG, Abouegylah M, Swaroop M, Skolny MN, Asdourian M, Gillespie T, Daniell K, Sayegh HE, Naoum GE, Zheng H, Taghian AG (2019) Timing of lymphedema after treatment for breast cancer: when are patients most at risk? Int J Radiat Oncol Biol Phys 103:62–70. https://doi.org/10.1016/j.ijrobp.2018.08.036
Miaskowski C, Dodd M, Paul SM, West C, Hamolsky D, Abrams G, Cooper BA, Elboim C, Neuhaus J, Schmidt BL, Smoot B, Aouizerat BE (2013) Lymphatic and angiogenic candidate genes predict the development of secondary lymphedema following breast cancer surgery. PLoS ONE 8:e60164. https://doi.org/10.1371/journal.pone.0060164
McLaughlin SA, Wright MJ, Morris KT, Giron GL, Sampson MR, Brockway JP, Hurley KE, Riedel ER, Van Zee KJ (2008) Prevalence of lymphedema in women with breast cancer 5 years after sentinel lymph node biopsy or axillary dissection: objective measurements. J Clin Oncol 26:5213–5219. https://doi.org/10.1200/JCO.2008.16.3725
Montagna G, Zhang J, Sevilimedu V, Charyn J, Abbate K, Gomez EA, Mehrara B, Morrow M, Barrio AV (2022) Risk factors and racial and ethnic disparities in patients with breast cancer-related lymphedema. JAMA Oncol 8:1195–1200. https://doi.org/10.1001/jamaoncol.2022.1628
Bucci LK, Brunelle CL, Bernstein MC, Shui AM, Gillespie TC, Roberts SA, Naoum GE, Taghian AG (2021) Subclinical lymphedema after treatment for breast cancer: risk of progression and considerations for early intervention. Ann Surg Oncol 28:8624–8633. https://doi.org/10.1245/s10434-021-10173-0
Whitworth P, Vicini F, Valente SA, Brownson K, DuPree B, Kohli M, Lawson L, Shah C (2022) Reducing rates of chronic breast cancer-related lymphedema with screening and early intervention: an update of recent data. J Cancer Surviv. https://doi.org/10.1007/s11764-022-01242-8
Koelmeyer LA, Borotkanics RJ, Alcorso J, Prah P, Winch CJ, Nakhel K, Dean CM, Boyages J (2019) Early surveillance is associated with less incidence and severity of breast cancer-related lymphedema compared with a traditional referral model of care. Cancer 125:854–862. https://doi.org/10.1002/cncr.31873
Ridner SH, Dietrich MS, Boyages J, Koelmeyer L, Elder E, Hughes TM, French J, Ngui N, Hsu J, Abramson VG, Moore A, Shah C (2022) A comparison of bioimpedance spectroscopy or tape measure triggered compression intervention in chronic breast cancer lymphedema prevention. Lymphat Res Biol. https://doi.org/10.1089/lrb.2021.0084
Aldrich MB, Rasmussen JC, DeSnyder SM, Woodward WA, Chan W, Sevick-Muraca EM, Mittendorf EA, Smith BD, Stauder MC, Strom EA, Perkins GH, Hoffman KE, Mitchell MP, Barcenas CH, Isales LE, Shaitelman SF (2022) Prediction of breast cancer-related lymphedema by dermal backflow detected with near-infrared fluorescence lymphatic imaging. Breast Cancer Res Treat 195:33–41. https://doi.org/10.1007/s10549-022-06667-4
Soran A, Ozmen T, McGuire KP, Diego EJ, McAuliffe PF, Bonaventura M, Ahrendt GM, DeGore L, Johnson R (2014) The importance of detection of subclinical lymphedema for the prevention of breast cancer-related clinical lymphedema after axillary lymph node dissection; a prospective observational study. Lymphat Res Biol 12:289–294. https://doi.org/10.1089/lrb.2014.0035
Whitworth PW, Shah C, Vicini F, Cooper A (2018) Preventing breast cancer-related lymphedema in high-risk patients: the impact of a structured surveillance protocol using bioimpedance spectroscopy. Front Oncol 8:197. https://doi.org/10.3389/fonc.2018.00197
Whitworth PW, Cooper A (2018) Reducing chronic breast cancer-related lymphedema utilizing a program of prospective surveillance with bioimpedance spectroscopy. Breast J 24:62–65. https://doi.org/10.1111/tbj.12939
Box RC, Reul-Hirche HM, Bullock-Saxton JE, Furnival CM (2002) Physiotherapy after breast cancer surgery: results of a randomised controlled study to minimise lymphoedema. Breast Cancer Res Treat 75:51–64. https://doi.org/10.1023/a:1016591121762
Kilgore LJ, Korentager SS, Hangge AN, Amin AL, Balanoff CR, Larson KE, Mitchell MP, Chen JG, Burgen E, Khan QJ, O’Dea AP, Nye L, Sharma P, Wagner JL (2018) Reducing breast cancer-related lymphedema (BCRL) through prospective surveillance monitoring using bioimpedance spectroscopy (BIS) and patient directed self-interventions. Ann Surg Oncol 25:2948–2952. https://doi.org/10.1245/s10434-018-6601-8
Torres Lacomba M, Yuste Sanchez MJ, Zapico Goni A, Prieto Merino D, Mayoral del Moral O, Cerezo Tellez E, Minayo Mogollon E (2010) Effectiveness of early physiotherapy to prevent lymphoedema after surgery for breast cancer: randomised, single blinded, clinical trial. BMJ 340:b5396. https://doi.org/10.1136/bmj.b5396
Paramanandam VS, Dylke E, Clark GM, Daptardar AA, Kulkarni AM, Nair NS, Badwe RA, Kilbreath SL (2022) Prophylactic use of compression sleeves reduces the incidence of arm swelling in women at high risk of breast cancer-related lymphedema: a randomized controlled trial. J Clin Oncol. https://doi.org/10.1200/JCO.21.02567
Armer JM, Radina ME, Porock D, Culbertson SD (2003) Predicting breast cancer-related lymphedema using self-reported symptoms. Nurs Res 52:370–379. https://doi.org/10.1097/00006199-200311000-00004
Norman SA, Localio AR, Potashnik SL, Simoes Torpey HA, Kallan MJ, Weber AL, Miller LT, Demichele A, Solin LJ (2009) Lymphedema in breast cancer survivors: incidence, degree, time course, treatment, and symptoms. J Clin Oncol 27:390–397. https://doi.org/10.1200/JCO.2008.17.9291
Rockson SG (2018) Lymphedema after breast cancer treatment. N Engl J Med 379:1937–1944. https://doi.org/10.1056/NEJMcp1803290
Michelotti A, Invernizzi M, Lopez G, Lorenzini D, Nesa F, De Sire A, Fusco N (2019) Tackling the diversity of breast cancer related lymphedema: perspectives on diagnosis, risk assessment, and clinical management. Breast 44:15–23. https://doi.org/10.1016/j.breast.2018.12.009
National Lymphedema Network (2012) NLN Position Paper: Lymphedema Risk Reduction Practices. https://static1.squarespace.com/static/5b741fa71aef1d1e6500b325/t/621c4aa760809f553732d386/1646021287719/risk_reduction_summary+.pdf. Accessed 16 Jan 2023
Harris SR, Schmitz KH, Campbell KL, McNeely ML (2012) Clinical practice guidelines for breast cancer rehabilitation: syntheses of guideline recommendations and qualitative appraisals. Cancer 118:2312–2324. https://doi.org/10.1002/cncr.27461
Borman P, Yaman A, Yasrebi S, Ozdemir O (2017) The importance of awareness and education in patients with breast cancer-related lymphedema. J Cancer Educ 32:629–633. https://doi.org/10.1007/s13187-016-1026-1
Ridner SH (2006) Pretreatment lymphedema education and identified educational resources in breast cancer patients. Patient Educ Couns 61:72–79. https://doi.org/10.1016/j.pec.2005.02.009
Martinez-Jaimez P, Fuster Linares P, Piller N, Masia J, Yamamoto T, Lopez-Montoya L, Monforte-Royo C (2022) Multidisciplinary preventive intervention for breast cancer-related lymphedema: an international consensus. Eur J Cancer Care (Engl). https://doi.org/10.1111/ecc.13704
Stout NL, Binkley JM, Schmitz KH, Andrews K, Hayes SC, Campbell KL, McNeely ML, Soballe PW, Berger AM, Cheville AL, Fabian C, Gerber LH, Harris SR, Johansson K, Pusic AL, Prosnitz RG, Smith RA (2012) A prospective surveillance model for rehabilitation for women with breast cancer. Cancer 118:2191–2200. https://doi.org/10.1002/cncr.27476
McLaughlin SA, Stout NL, Schaverien MV (2020) Avoiding the swell: advances in lymphedema prevention, detection, and management. Am Soc Clin Oncol Educ Book 40:1–10. https://doi.org/10.1200/EDBK_280471
Stout NL, Pfalzer LA, Springer B, Levy E, McGarvey CL, Danoff JV, Gerber LH, Soballe PW (2012) Breast cancer-related lymphedema: comparing direct costs of a prospective surveillance model and a traditional model of care. Phys Ther 92:152–163. https://doi.org/10.2522/ptj.20100167
Brunelle C, Skolny M, Ferguson C, Swaroop M, O’Toole J, Taghian AG (2015) Establishing and sustaining a prospective screening program for breast cancer-related lymphedema at the massachusetts general hospital: lessons learned. J Pers Med 5:153–164. https://doi.org/10.3390/jpm5020153
Koelmeyer L, Gaitatzis K, Ridner SH, Boyages J, Nelms J, Hughes TM, Elder E, French J, Ngui N, Hsu J, Stolldorf D (2021) Implementing a prospective surveillance and early intervention model of care for breast cancer-related lymphedema into clinical practice: application of the RE-AIM framework. Support Care Cancer 29:1081–1089. https://doi.org/10.1007/s00520-020-05597-5
Koelmeyer LA, Moloney E, Boyages J, Sherman KA, Dean CM (2021) Prospective surveillance model in the home for breast cancer-related lymphoedema: a feasibility study. Breast Cancer Res Treat 185:401–412. https://doi.org/10.1007/s10549-020-05953-3
Thompson M, Korourian S, Henry-Tillman R, Adkins L, Mumford S, Westbrook KC, Klimberg VS (2007) Axillary reverse mapping (ARM): a new concept to identify and enhance lymphatic preservation. Ann Surg Oncol 14:1890–1895. https://doi.org/10.1245/s10434-007-9412-x
Tummel E, Ochoa D, Korourian S, Betzold R, Adkins L, McCarthy M, Hung S, Kalkwarf K, Gallagher K, Lee JY, Klimberg VS (2017) Does axillary reverse mapping prevent lymphedema after lymphadenectomy? Ann Surg 265:987–992. https://doi.org/10.1097/SLA.0000000000001778
Ahmed M, Rubio IT, Kovacs T, Klimberg VS, Douek M (2016) Systematic review of axillary reverse mapping in breast cancer. Br J Surg 103:170–178. https://doi.org/10.1002/bjs.10041
Han C, Yang B, Zuo WS, Zheng G, Yang L, Zheng MZ (2016) The feasibility and oncological safety of axillary reverse mapping in patients with breast cancer: a systematic review and meta-analysis of prospective studies. PLoS ONE 11:e0150285. https://doi.org/10.1371/journal.pone.0150285
Boccardo F, Casabona F, De Cian F, Friedman D, Villa G, Bogliolo S, Ferrero S, Murelli F, Campisi C (2009) Lymphedema microsurgical preventive healing approach: a new technique for primary prevention of arm lymphedema after mastectomy. Ann Surg Oncol 16:703–708. https://doi.org/10.1245/s10434-008-0270-y
McLaughlin SA, DeSnyder SM, Klimberg S, Alatriste M, Boccardo F, Smith ML, Staley AC, Thiruchelvam PTR, Hutchison NA, Mendez J, MacNeill F, Vicini F, Rockson SG, Feldman SM (2017) Considerations for clinicians in the diagnosis, prevention, and treatment of breast cancer-related lymphedema, recommendations from an expert panel: part 2: preventive and therapeutic options. Ann Surg Oncol 24:2827–2835. https://doi.org/10.1245/s10434-017-5964-6
Boccardo FM, Casabona F, Friedman D, Puglisi M, De Cian F, Ansaldi F, Campisi C (2011) Surgical prevention of arm lymphedema after breast cancer treatment. Ann Surg Oncol 18:2500–2505. https://doi.org/10.1245/s10434-011-1624-4
Hyland CJ, Manrique OJ, Weiss A, Broyles JM (2022) Preventive strategies for breast cancer-related lymphedema: working toward optimal patient selection. Cancer 128:3284–3286. https://doi.org/10.1002/cncr.34374
DeSnyder SM, Yi M, Boccardo F, Feldman S, Klimberg VS, Smith M, Thiruchelvam PTR, McLaughlin S (2021) American Society of Breast Surgeons’ practice patterns for patients at risk and affected by breast cancer-related lymphedema. Ann Surg Oncol 28:5742–5751. https://doi.org/10.1245/s10434-021-10494-0
Ozmen T, Layton C, Friedman-Eldar O, Melnikau S, Kesmodel S, Moller MG, Avisar E (2022) Evaluation of simplified lymphatic microsurgical preventing healing approach (SLYMPHA) for the prevention of breast cancer-related lymphedema after axillary lymph node dissection using bioimpedance spectroscopy. Eur J Surg Oncol 48:1713–1717. https://doi.org/10.1016/j.ejso.2022.04.023
Rockson SG, Keeley V, Kilbreath S, Szuba A, Towers A (2019) Cancer-associated secondary lymphoedema. Nat Rev Dis Primers 5:22. https://doi.org/10.1038/s41572-019-0072-5
Executive Committee of the International Society of L (2020) The diagnosis and treatment of peripheral lymphedema: 2020 Consensus Document of the International Society of Lymphology. Lymphology 53:3–19
Lasinski BB, McKillip Thrift K, Squire D, Austin MK, Smith KM, Wanchai A, Green JM, Stewart BR, Cormier JN, Armer JM (2012) A systematic review of the evidence for complete decongestive therapy in the treatment of lymphedema from 2004 to 2011. PM R 4:580–601. https://doi.org/10.1016/j.pmrj.2012.05.003
Foldi M (2006) Chapter 11. Complete decongestive therapy. In: Foldi MF, Strossenreuther E, Kubiks RS (eds) Foldi’s textbook of lymphology for physicians and lymphedema therapiests. Elsevier, Munich
Davies C, Levenhagen K, Ryans K, Perdomo M, Gilchrist L (2020) Interventions for breast cancer-related lymphedema: clinical practice guideline from the academy of oncologic physical therapy of APTA. Phys Ther 100:1163–1179. https://doi.org/10.1093/ptj/pzaa087
Lane K, Worsley D, McKenzie D (2005) Exercise and the lymphatic system: implications for breast-cancer survivors. Sports Med 35:461–471. https://doi.org/10.2165/00007256-200535060-00001
Devoogdt N, Van Kampen M, Geraerts I, Coremans T, Christiaens MR (2010) Different physical treatment modalities for lymphoedema developing after axillary lymph node dissection for breast cancer: a review. Eur J Obstet Gynecol Reprod Biol 149:3–9. https://doi.org/10.1016/j.ejogrb.2009.11.016
Suami H, Heydon-White A, Mackie H, Czerniec S, Koelmeyer L, Boyages J (2019) A new indocyanine green fluorescence lymphography protocol for identification of the lymphatic drainage pathway for patients with breast cancer-related lymphoedema. BMC Cancer 19:985. https://doi.org/10.1186/s12885-019-6192-1
Koelmeyer LA, Thompson BM, Mackie H, Blackwell R, Heydon-White A, Moloney E, Gaitatzis K, Boyages J, Suami H (2021) Personalizing conservative lymphedema management using indocyanine green-guided manual lymphatic drainage. Lymphat Res Biol 19:56–65. https://doi.org/10.1089/lrb.2020.0090
Perdomo M, Davies C, Levenhagen K, Ryans K, Gilchrist L (2022) Patient education for breast cancer-related lymphedema: a systematic review. J Cancer Surviv. https://doi.org/10.1007/s11764-022-01262-4
Hasenoehrl T, Palma S, Ramazanova D, Kolbl H, Dorner TE, Keilani M, Crevenna R (2020) Resistance exercise and breast cancer-related lymphedema-a systematic review update and meta-analysis. Support Care Cancer 28:3593–3603. https://doi.org/10.1007/s00520-020-05521-x
Rogan S, Taeymans J, Luginbuehl H, Aebi M, Mahnig S, Gebruers N (2016) Therapy modalities to reduce lymphoedema in female breast cancer patients: a systematic review and meta-analysis. Breast Cancer Res Treat 159:1–14. https://doi.org/10.1007/s10549-016-3919-4
Vignes S, Porcher R, Arrault M, Dupuy A (2007) Long-term management of breast cancer-related lymphedema after intensive decongestive physiotherapy. Breast Cancer Res Treat 101:285–290. https://doi.org/10.1007/s10549-006-9297-6
Paskett ED, Le-Rademacher J, Oliveri JM, Liu H, Seisler DK, Sloan JA, Armer JM, Naughton MJ, Hock K, Schwartz M, Unzeitig G, Melnik M, Yee LD, Fleming GF, Taylor JR, Loprinzi C (2021) A randomized study to prevent lymphedema in women treated for breast cancer: CALGB 70305 (Alliance). Cancer 127:291–299. https://doi.org/10.1002/cncr.33183
Ezzo J, Manheimer E, McNeely ML, Howell DM, Weiss R, Johansson KI, Bao T, Bily L, Tuppo CM, Williams AF, Karadibak D (2015) Manual lymphatic drainage for lymphedema following breast cancer treatment. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD003475.pub2
Thompson B, Gaitatzis K, Janse de Jonge X, Blackwell R, Koelmeyer LA (2021) Manual lymphatic drainage treatment for lymphedema: a systematic review of the literature. J Cancer Surviv 15:244–258. https://doi.org/10.1007/s11764-020-00928-1
Tambour M, Holt M, Speyer A, Christensen R, Gram B (2018) Manual lymphatic drainage adds no further volume reduction to Complete Decongestive Therapy on breast cancer-related lymphoedema: a multicentre, randomised, single-blind trial. Br J Cancer 119:1215–1222. https://doi.org/10.1038/s41416-018-0306-4
Andersen L, Hojris I, Erlandsen M, Andersen J (2000) Treatment of breast-cancer-related lymphedema with or without manual lymphatic drainage—a randomized study. Acta Oncol 39:399–405. https://doi.org/10.1080/028418600750013186
Gradalski T, Ochalek K, Kurpiewska J (2015) Complex Decongestive Lymphatic Therapy with or without Vodder II manual lymph drainage in more severe chronic postmastectomy upper limb lymphedema: a randomized noninferiority prospective study. J Pain Symptom Manag 50:750–757. https://doi.org/10.1016/j.jpainsymman.2015.06.017
Huang TW, Tseng SH, Lin CC, Bai CH, Chen CS, Hung CS, Wu CH, Tam KW (2013) Effects of manual lymphatic drainage on breast cancer-related lymphedema: a systematic review and meta-analysis of randomized controlled trials. World J Surg Oncol 11:15. https://doi.org/10.1186/1477-7819-11-15
Lin Y, Yang Y, Zhang X, Li W, Li H, Mu D (2022) Manual lymphatic drainage for breast cancer-related lymphedema: a systematic review and meta-analysis of randomized controlled trials. Clin Breast Cancer. https://doi.org/10.1016/j.clbc.2022.01.013
De Vrieze T, Gebruers N, Nevelsteen I, Thomis S, De Groef A, Tjalma WAA, Belgrado JP, Vandermeeren L, Monten C, Hanssens M, Asnong A, Dams L, Van der Gucht E, Heroes AK, Devoogdt N (2022) Does manual lymphatic drainage add value in reducing arm volume in patients with breast cancer-related lymphedema? Phys Ther. https://doi.org/10.1093/ptj/pzac137
Lawenda BD, Mondry TE, Johnstone PA (2009) Lymphedema: a primer on the identification and management of a chronic condition in oncologic treatment. CA Cancer J Clin 59:8–24. https://doi.org/10.3322/caac.20001
Pappalardo M, Starnoni M, Franceschini G, Baccarani A, De Santis G (2021) Breast cancer-related lymphedema: recent updates on diagnosis, severity and available treatments. J Pers Med. https://doi.org/10.3390/jpm11050402
Finkelstein ER, Ha M, Hanwright P, Ngaage LM, Yoon JS, Liang F, Nam AJ, Rasko YM (2022) A review of American insurance coverage and criteria for the conservative management of lymphedema. J Vasc Surg Venous Lymphat Disord. https://doi.org/10.1016/j.jvsv.2022.03.008
Smile TD, Tendulkar R, Schwarz G, Arthur D, Grobmyer S, Valente S, Vicini F, Shah C (2018) A review of treatment for breast cancer-related lymphedema: paradigms for clinical practice. Am J Clin Oncol 41:178–190. https://doi.org/10.1097/COC.0000000000000355
Lasinski BB (2013) Complete decongestive therapy for treatment of lymphedema. Semin Oncol Nurs 29:20–27. https://doi.org/10.1016/j.soncn.2012.11.004
Koul R, Dufan T, Russell C, Guenther W, Nugent Z, Sun X, Cooke AL (2007) Efficacy of complete decongestive therapy and manual lymphatic drainage on treatment-related lymphedema in breast cancer. Int J Radiat Oncol Biol Phys 67:841–846. https://doi.org/10.1016/j.ijrobp.2006.09.024
Dayes IS, Whelan TJ, Julian JA, Parpia S, Pritchard KI, D’Souza DP, Kligman L, Reise D, LeBlanc L, McNeely ML, Manchul L, Wiernikowski J, Levine MN (2013) Randomized trial of decongestive lymphatic therapy for the treatment of lymphedema in women with breast cancer. J Clin Oncol 31:3758–3763. https://doi.org/10.1200/JCO.2012.45.7192
Godette K, Mondry TE, Johnstone PA (2006) Can manual treatment of lymphedema promote metastasis? J Soc Integr Oncol 4:8–12
Szuba A, Achalu R, Rockson SG (2002) Decongestive lymphatic therapy for patients with breast carcinoma-associated lymphedema. A randomized, prospective study of a role for adjunctive intermittent pneumatic compression. Cancer 95:2260–2267. https://doi.org/10.1002/cncr.10976
Feldman JL, Stout NL, Wanchai A, Stewart BR, Cormier JN, Armer JM (2012) Intermittent pneumatic compression therapy: a systematic review. Lymphology 45:13–25
Rockson SG, Whitworth PW, Cooper A, Kania S, Karnofel H, Nguyen M, Shadduck K, Gingerich P, Armer J (2022) Safety and effectiveness of a novel nonpneumatic active compression device for treating breast cancer-related lymphedema: a multicenter randomized, crossover trial (NILE). J Vasc Surg Venous Lymphat Disord. https://doi.org/10.1016/j.jvsv.2022.06.016
Baxter GD, Liu L, Petrich S, Gisselman AS, Chapple C, Anders JJ, Tumilty S (2017) Low level laser therapy (photobiomodulation therapy) for breast cancer-related lymphedema: a systematic review. BMC Cancer 17:833. https://doi.org/10.1186/s12885-017-3852-x
Mayrovitz HN, Davey S (2011) Changes in tissue water and indentation resistance of lymphedematous limbs accompanying low level laser therapy (LLLT) of fibrotic skin. Lymphology 44:168–177
Piller NB, Thelander A (1998) Treatment of chronic postmastectomy lymphedema with low level laser therapy: a 2.5 year follow-up. Lymphology 31:74–86
Carati CJ, Anderson SN, Gannon BJ, Piller NB (2003) Treatment of postmastectomy lymphedema with low-level laser therapy: a double blind, placebo-controlled trial. Cancer 98:1114–1122. https://doi.org/10.1002/cncr.11641
Lau RW, Cheing GL (2009) Managing postmastectomy lymphedema with low-level laser therapy. Photomed Laser Surg 27:763–769. https://doi.org/10.1089/pho.2008.2330
Ahmed Omar MT, Abd-El-Gayed Ebid A, El Morsy AM (2011) Treatment of post-mastectomy lymphedema with laser therapy: double blind placebo control randomized study. J Surg Res 165:82–90. https://doi.org/10.1016/j.jss.2010.03.050
Ridner SH, Poage-Hooper E, Kanar C, Doersam JK, Bond SM, Dietrich MS (2013) A pilot randomized trial evaluating low-level laser therapy as an alternative treatment to manual lymphatic drainage for breast cancer-related lymphedema. Oncol Nurs Forum 40:383–393. https://doi.org/10.1188/13.ONF.383-393
Mahmood D, Ahmad A, Sharif F, Arslan SA (2022) Clinical application of low-level laser therapy (photo-biomodulation therapy) in the management of breast cancer-related lymphedema: a systematic review. BMC Cancer 22:937. https://doi.org/10.1186/s12885-022-10021-8
Smoot B, Chiavola-Larson L, Lee J, Manibusan H, Allen DD (2015) Effect of low-level laser therapy on pain and swelling in women with breast cancer-related lymphedema: a systematic review and meta-analysis. J Cancer Surviv 9:287–304. https://doi.org/10.1007/s11764-014-0411-1
Mt EL, Jg EL, de Andrade MF, Bergmann A (2014) Low-level laser therapy in secondary lymphedema after breast cancer: systematic review. Lasers Med Sci 29:1289–1295. https://doi.org/10.1007/s10103-012-1240-y
Chang EI, Skoracki RJ, Chang DW (2018) Lymphovenous anastomosis bypass surgery. Semin Plast Surg 32:22–27. https://doi.org/10.1055/s-0038-1636510
Chang DW, Suami H, Skoracki R (2013) A prospective analysis of 100 consecutive lymphovenous bypass cases for treatment of extremity lymphedema. Plast Reconstr Surg 132:1305–1314. https://doi.org/10.1097/PRS.0b013e3182a4d626
AlJindan FK, Lin CY, Cheng MH (2019) Comparison of outcomes between side-to-end and end-to-end lymphovenous anastomoses for early-grade extremity lymphedema. Plast Reconstr Surg 144:486–496. https://doi.org/10.1097/PRS.0000000000005870
Suami H, Chang DW (2012) New developments in microsurgery. In: Moffat C (ed) Lymphedema framework: best practice for the management of Lymphoedema. Surgical intervention. A position document on surgery for Lymphedema, 2 edn. The International Lymphoedema Framework and World Alliance for Wound and Lymphedema Care, Saint Étienne, pp 34–35
Schaverien MV, Asaad M, Selber JC, Liu J, Chen DN, Hall MS, Butler CE (2021) Outcomes of vascularized lymph node transplantation for treatment of lymphedema. J Am Coll Surg 232:982–994. https://doi.org/10.1016/j.jamcollsurg.2021.03.002
Granzow JW, Soderberg JM, Kaji AH, Dauphine C (2014) Review of current surgical treatments for lymphedema. Ann Surg Oncol 21:1195–1201. https://doi.org/10.1245/s10434-014-3518-8
Ozturk CN, Ozturk C, Glasgow M, Platek M, Ashary Z, Kuhn J, Aronoff N, Lohman R, Djohan R, Gurunluoglu R (2016) Free vascularized lymph node transfer for treatment of lymphedema: a systematic evidence based review. J Plast Reconstr Aesthet Surg 69:1234–1247. https://doi.org/10.1016/j.bjps.2016.06.022
Winters H, Tielemans HJP, Paulus V, Hummelink S, Slater NJ, Ulrich DJO (2022) A systematic review and meta-analysis of vascularized lymph node transfer for breast cancer-related lymphedema. J Vasc Surg Venous Lymphat Disord 10:786-797e781. https://doi.org/10.1016/j.jvsv.2021.08.023
Ngo QD, Munot S, Mackie H, Czerniec S, Koelmeyer LA, Lam T, Heydon-White A, Suami H, Boyages J (2020) Vascularized lymph node transfer for patients with breast cancer-related lymphedema can potentially reduce the burden of ongoing conservative management. Lymphat Res Biol 18:357–364. https://doi.org/10.1089/lrb.2019.0048
Boyages J, Kalfa S, Xu Y, Koelmeyer L, Mackie H, Viveros H, Taksa L, Gollan P (2016) Worse and worse off: the impact of lymphedema on work and career after breast cancer. Springerplus 5:657. https://doi.org/10.1186/s40064-016-2300-8
Boyages J, Xu Y, Kalfa S, Koelmeyer L, Parkinson B, Mackie H, Viveros H, Gollan P, Taksa L (2017) Financial cost of lymphedema borne by women with breast cancer. Psychooncology 26:849–855. https://doi.org/10.1002/pon.4239
Vignes S, Blanchard M, Yannoutsos A, Arrault M (2013) Complications of autologous lymph-node transplantation for limb lymphoedema. Eur J Vasc Endovasc Surg 45:516–520. https://doi.org/10.1016/j.ejvs.2012.11.026
Brorson H (2003) Liposuction in arm lymphedema treatment. Scand J Surg 92:287–295. https://doi.org/10.1177/145749690309200409
Schaverien MV, Munnoch DA, Brorson H (2018) Liposuction treatment of lymphedema. Semin Plast Surg 32:42–47. https://doi.org/10.1055/s-0038-1635116
Boyages J, Kastanias K, Koelmeyer LA, Winch CJ, Lam TC, Sherman KA, Munnoch DA, Brorson H, Ngo QD, Heydon-White A, Magnussen JS, Mackie H (2015) Liposuction for advanced lymphedema: a multidisciplinary approach for complete reduction of arm and leg swelling. Ann Surg Oncol 22(Suppl 3):S1263-1270. https://doi.org/10.1245/s10434-015-4700-3
Brorson H (2000) Liposuction gives complete reduction of chronic large arm lymphedema after breast cancer. Acta Oncol 39:407–420. https://doi.org/10.1080/028418600750013195
Schaverien MV, Munro KJ, Baker PA, Munnoch DA (2012) Liposuction for chronic lymphoedema of the upper limb: 5 years of experience. J Plast Reconstr Aesthet Surg 65:935–942. https://doi.org/10.1016/j.bjps.2012.01.021
Damstra RJ, Voesten HG, Klinkert P, Brorson H (2009) Circumferential suction-assisted lipectomy for lymphoedema after surgery for breast cancer. Br J Surg 96:859–864. https://doi.org/10.1002/bjs.6658
Lee D, Piller N, Hoffner M, Manjer J, Brorson H (2016) Liposuction of postmastectomy arm lymphedema decreases the incidence of erysipelas. Lymphology 49:85–92
Hoffner M, Bagheri S, Hansson E, Manjer J, Troeng T, Brorson H (2017) SF-36 shows increased quality of life following complete reduction of postmastectomy lymphedema with liposuction. Lymphat Res Biol 15:87–98. https://doi.org/10.1089/lrb.2016.0035
Armer JM, Stewart BR (2010) Post-breast cancer lymphedema: incidence increases from 12 to 30 to 60 months. Lymphology 43:118–127
Schwarz GS, Grobmyer SR, Djohan RS, Cakmakoglu C, Bernard SL, Radford D, Al-Hilli Z, Knackstedt R, Djohan M, Valente SA (2019) Axillary reverse mapping and lymphaticovenous bypass: lymphedema prevention through enhanced lymphatic visualization and restoration of flow. J Surg Oncol 120:160–167. https://doi.org/10.1002/jso.25513
Shaffer K, Cakmakoglu C, Schwarz GS, ElSherif A, Al-Hilli Z, Djohan R, Radford DM, Grobmyer S, Bernard S, Moreira A, Fanning A, Tu C, Valente SA (2020) Lymphedema prevention surgery: improved operating efficiency over time. Ann Surg Oncol 27:4695–4701. https://doi.org/10.1245/s10434-020-08890-z
Rockson SG, Tian W, Jiang X, Kuznetsova T, Haddad F, Zampell J, Mehrara B, Sampson JP, Roche L, Kim J, Nicolls MR (2018) Pilot studies demonstrate the potential benefits of antiinflammatory therapy in human lymphedema. JCI Insight. https://doi.org/10.1172/jci.insight.123775
Chen MC, Korth CC, Harnett MD, Elenko E, Lickliter JD (2022) A randomized phase 1 evaluation of deupirfenidone, a novel deuterium-containing drug candidate for interstitial lung disease and other inflammatory and fibrotic diseases. Clin Pharmacol Drug Dev 11:220–234. https://doi.org/10.1002/cpdd.1040
Hartiala P, Suominen S, Suominen E, Kaartinen I, Kiiski J, Viitanen T, Alitalo K, Saarikko AM (2020) Phase 1 Lymfactin(®) study: short-term safety of combined adenoviral VEGF-C and lymph node transfer treatment for upper extremity lymphedema. J Plast Reconstr Aesthet Surg 73:1612–1621. https://doi.org/10.1016/j.bjps.2020.05.009
Hartiala P, Lahdenperä O, Vuolanto A, Saarikko A (2020) Abstract OT1-06-01: Lymfactin, an investigational adenoviral gene therapy expressing VEGF-C, is currently studied in a double-blind, randomized, placebo-controlled, multicenter, phase 2 clinical study in patients suffering from breast cancer associated secondary lymphedema (BCAL). Cancer Res 80:OT1-06-01-OT01-06–01. https://doi.org/10.1158/1538-7445.Sabcs19-ot1-06-01
Herantis Pharma Plc (2021) Herantis announces inconclusive results from phase II study with Lymfactin in breast cancer related lymphedema. https://herantis.com/press-releases/herantis-announces-inconclusive-results-from-phase-ii-study-with-lymfactin-in-breast-cancer-related-lymphedema/. Accessed 16 Jan 2023
Mehrara BJ, Park HJ, Kataru RP, Bromberg J, Coriddi M, Baik JE, Shin J, Li C, Cavalli MR, Encarnacion EM, Lee M, Van Zee KJ, Riedel E, Dayan JH (2021) Pilot study of anti-Th2 immunotherapy for the treatment of breast cancer-related upper extremity lymphedema. Biology (Basel). https://doi.org/10.3390/biology10090934
Acknowledgements
We would like to thank Robert Rydzewski and Mary Susan Prescott (Prescott Medical Communications Group; Chicago, IL) and Carol Cheli (Clinical & Medical Affairs Consulting, LLC; West Palm Beach, FL), who provided medical writing assistance, with financial support from PureTech Health, plc.
Funding
Financial support for this manuscript was provided by PureTech Health plc (Boston, MA).
Author information
Authors and Affiliations
Contributions
All authors participated in conception of the analysis, data review, and critical review of the manuscript. All authors read and approval for the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Financial Interests: P.M.C.D. is a paid consultant for PureTech Health and Tactile Medical and has received clinical and imaging grants from LymphaTouch, Inc. A.M. is a paid clinical advisor to AIROS Medical, Inc. and a paid consultant for PureTech Health. A.F. is a full-time employee of PureTech Health. L.K. has received research support from PureTech Health.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Donahue, P.M.C., MacKenzie, A., Filipovic, A. et al. Advances in the prevention and treatment of breast cancer-related lymphedema. Breast Cancer Res Treat 200, 1–14 (2023). https://doi.org/10.1007/s10549-023-06947-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-023-06947-7