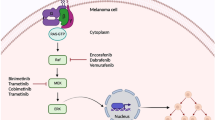
Abstract
Purpose
Rash develops in approximately 50% of patients receiving alpelisib for breast cancer, often requiring dose modifications. Here, we describe the clinicopathologic, laboratory, and management characteristics of alpelisib-related dermatologic adverse events (dAEs).
Methods
A single center-retrospective analysis was conducted. Data were abstracted from electronic medical records.
Results
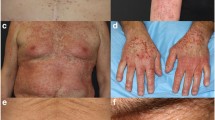
A total of 102 patients (mean age 56 years, range 27–83) receiving alpelisib most frequently in combination with endocrine therapy (79, 77.5%) were included. We identified 41 (40.2%) patients with all-grade rash distributed primarily along the trunk (78%) and extremities (70%) that developed approximately within two weeks of treatment initiation (mean 12.8 ± 1.5 days) and lasted one-week (mean duration 7.1 ± 0.8 days). Of 29 patients with documented morphology of alpelisib-related dAEs, 26 (89.7%) had maculopapular rash. Histology showed perivascular and interface lymphocytic dermatitis. All-grade rash correlated with an increase in serum eosinophils from 2.7 to 4.4%, p < 0.05, and prophylaxis with non-sedating antihistamines (n = 43) was correlated with a reduction of grade 1/2 rash (OR 0.39, p = 0.09). Sixteen (84.2%) of 19 patients with grade 3 dAEs resulted in interruption of alpelisib, which were managed with antihistamines, topical and systemic corticosteroids. We did not observe rash recurrence in 12 (75%) patients who were re-challenged.
Conclusions
A maculopapular rash associated with increased blood eosinophils occurs frequently with alpelisib. While grade 3 rash leads to alpelisib therapy interruption, dermatologic improvement is evident with systemic corticosteroids; and most patients can continue oncologic treatment at a maintained or reduced dose upon re-challenge with alpelisib.




Similar content being viewed by others
Abbreviations
- ALT:
-
Alanine aminotransferase
- BYL719:
-
Alpelisib
- CBC:
-
Complete blood count
- CTCAE:
-
Common Terminology Criteria for Adverse Events
- dAEs:
-
Dermatologic adverse events
- DRESS:
-
Drug reaction with eosinophilia and systemic symptoms
- EGFR:
-
Epidermal growth factor receptor
- EMR:
-
Electronic medical record
- HbA1C:
-
Glycohemoglobin
- HER2:
-
Human epidermal growth factor receptor-2
- HER3:
-
Human epidermal growth factor receptor-3
- HR:
-
Hormone receptor
- mAb:
-
Monoclonal antibody
- MEK:
-
Mitogen-activated protein kinase enzyme
- OR:
-
Odds ratio
- PI3K:
-
Phosphatidylinositol-3-kinase
- PIK3CA:
-
Phosphatidylinositol-4,5-phosphate-3-kinase alpha catalytic subunit
- PTEN:
-
Phosphatase and tensin homolog
- RTK:
-
Receptor tyrosine kinase
- SEM:
-
Standard error mean
- SERD:
-
Selective estrogen receptor degrader
- SERM:
-
Selective estrogen receptor modulator
- SJS/TEN:
-
Stevens–Johnson syndrome/toxic epidermal necrolysis
References
Miller TW, Rexer BN, Garrett JT, Arteaga CL (2011) Mutations in the phosphatidylinositol 3-kinase pathway: role in tumor progression and therapeutic implications in breast cancer. Breast Cancer Res 13:224
Zardavas D, Marvelde L, Milne RL, Fumagalli D, Fountzilas G, Kotoula V, Razis E, Papaxoinis G, Joensuu H, Moynahan ME, Hennessy BT, Bieche I, Saal LH, Stal O, Iacopetta B, Jensen JD, O’Toole S, Lopez-Knowles E, Barbaraeschi M, Noguchi S, Azim HA, Lerma E, Bachelot T, Wang Q, Perez-Tenorio G, de Velde CJH, Rea DW, Sabine V, Bartlett JMS, Sotiriou C, Michiels S, Loi S (2018) Tumor PIK3CA genotype and prognosis in early-stage breast cancer: a pooled analysis of individual patient data. J Clin Oncol 36(10):981–989. https://doi.org/10.1200/JCO.2017.74.8301
Razavi P, Chang MT, Xu G, Bandlamudi C, Ross DS, Vasan N, Cai Y, Bielski CM, Donoghue MTA, Jonsson P, Penson A, Shen R, Pareja F, Kundra R, Middha S, Cheng ML, Zehir A, Kandoth C, Patel R, Huberman K, Smyth LM, Jhaveri K, Modi S, Traina TA, Dang C, Zhang W, Weigelt B, Li BT, Ladanyi M, Hyman DM, Schultz N, Robson ME, Hudis C, Brogi E, Viale A, Norton L, Dickler MN, Berger MF, Iacobuzio-Donahue CA, Chandarlapaty S, Scaltriti M, Reis-Filho JS, Solit DB, Taylor BS, Baselga J (2018) The genomic landscape of endocrine-resistant advanced breast cancers. Cancer Cell 34(3):427–438. https://doi.org/10.1016/j.ccell.2018.08.008
Andre F, Ciruelos E, Rubovszky G, Campone M, Loibl S, Rugo HS, Iwata H, Conte P, Mayer IA, Kaufman B, Yamashita T, Lu YS, Inoue K, Takahashi M, Papai Z, Longin AS, Mills D, Wilke C, Hirawat S, Juric D, Group S-S (2019) Alpelisib for PIK3CA-mutated, hormone receptor-positive advanced breast cancer. N Engl J Med 380(20):1929–1940. https://doi.org/10.1056/NEJMoa1813904
Juric D, Janku F, Rodon J, Burris HA, Mayer IA, Schuler M, Seggewiss-Bernhardt R, Gil-Martin M, Middleton MR, Baselga J, Bootle D, Demanse D, Blumenstein L, Schumacher K, Huang A, Quadt C, Rugo HS (2019) Alpelisib plus fulvestrant in PIK3CA-altered and PIK3CA-wild-type estrogen receptor-positive advanced breast cancer: a phase 1b clinical trial. JAMA Oncol 5(2):e184475. https://doi.org/10.1001/jamaoncol.2018.4475
Juric D, Rodon J, Tabernero J, Janku F, Burris HA, Schellens JHM, Middleton MR, Berlin J, Schuler M, Gil-Martin M, Rugo HS, Seggewiss-Bernhardt R, Huang A, Bootle D, Blumenstein L, Coughlin C, Quadt C, Baselga J (2018) Phosphatidylinositol 3 kinase alpha-selective inhibition with alpelisib (BYL719) in PIK3CA-altered solid tumors: results from the first in-human study. J Clin Oncol 36:1–9. https://doi.org/10.1200/JCO
Mayer IA, Abramson VG, Formisano L, Balko JM, Estrada MV, Sanders ME, Juric D, Solit D, Berger MF, Won HH, Li Y, Cantley LC, Winer E, Arteaga CL (2017) A phase Ib study of alpelisib (BYL719), a PI3Kalpha-specific inhibitor, with Letrozole in ER+/HER2- metastatic breast cancer. Clin Cancer Res 23(1):26–34. https://doi.org/10.1158/1078-0432.CCR-16-0134
Ando Y, Iwasa S, Takahashi S, Saka H, Kakizume T, Natsume K, Suenaga N, Quadt C, Yamada Y (2019) Phase I study of alpelisib (BYL719), an alpha-specific PI3K inhibitor, in Japanese patients with advanced solid tumors. Cancer Sci 110(3):1021–1031. https://doi.org/10.1111/cas.13923
Sheu J, Hawryluk EB, Litsas G, Thakuria M, LeBoeuf NR (2015) Papulopustular acneiform eruptions resulting from trastuzumab, a HER2 inhibitor. Clin Breast Cancer 15(1):e77–81. https://doi.org/10.1016/j.clbc.2014.09.003
Shiohara T, Mizukawa Y (2019) Drug-induced hypersensitivity syndrome (DiHS)/drug reaction with eosinophilia and systemic symptoms (DRESS): an update in 2019. Allergol Int 68(3):301–308. https://doi.org/10.1016/j.alit.2019.03.006
Lacouture M, Sibaud V (2018) Toxic side effects of targeted therapies and immunotherapies affecting the skin, oral mucosa, hair, and nails. Am J Clin Dermatol 19(1):31–39. https://doi.org/10.1007/s40257-018-0384-3
Curry JL, Torres-Cabala CA, Kim KB, Tetzlaff MT, Duvic M, Tsai KY, Hong DS, Prieto VG (2014) Dermatologic toxicities to targeted cancer therapy: shared clinical and histologic adverse skin reactions. Int J Dermatol 53:376–384
Bruce I, Akhlaq M, Bloomfield GC, Budd E, Cox B, Cuenoud B, Finan P, Gedeck P, Hatto J, Hayler JF, Head D, Keller T, Kirman L, Leblanc C, Le Grand D, McCarthy C, O'Connor D, Owen C, Oza MS, Pilgrim G, Press NE, Sviridenko L, Whitehead L (2012) Development of isoform selective PI3-kinase inhibitors as pharmacological tools for elucidating the PI3K pathway. Bioorg Med Chem Lett 22(17):5445–5450. https://doi.org/10.1016/j.bmcl.2012.07.042
Bendell JC, Rodon J, Burris HA, de Jonge M, Verweij J, Birle D, Demanse D, De Buck SS, Ru QC, Peters M, Goldbrunner M, Baselga J (2012) Phase I, dose-escalation study of BKM120, an oral pan-Class I PI3K inhibitor, in patients with advanced solid tumors. J Clin Oncol 30(3):282–290. https://doi.org/10.1200/JCO.2011.36.1360
Baselga J, Im S-A, Iwata H, Cortés J, De Laurentiis M, Jiang Z, Arteaga CL, Jonat W, Clemons M, Ito Y, Awada A, Chia S, Jagiełło-Gruszfeld A, Pistilli B, Tseng L-M, Hurvitz S, Masuda N, Takahashi M, Vuylsteke P, Hachemi S, Dharan B, Di Tomaso E, Urban P, Massacesi C, Campone M (2017) Buparlisib plus fulvestrant versus placebo plus fulvestrant in postmenopausal, hormone receptor-positive, HER2-negative, advanced breast cancer (BELLE-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 18(7):904–916. https://doi.org/10.1016/s1470-2045(17)30376-5
Gopal AK, Kahl BS, de Vos S, Wagner-Johnston ND, Schuster SJ, Jurczak WJ, Flinn IW, Flowers CR, Martin P, Viardot A, Blum KA, Goy AH, Davies AJ, Zinzani PL, Dreyling M, Johnson D, Miller LL, Holes L, Li D, Dansey RD, Godfrey WR, Salles GA (2014) PI3Kdelta inhibition by idelalisib in patients with relapsed indolent lymphoma. N Engl J Med 370(11):1008–1018. https://doi.org/10.1056/NEJMoa1314583
Sharman JP, Coutre SE, Furman RR, Cheson BD, Pagel JM, Hillmen P, Barrientos JC, Zelenetz AD, Kipps TJ, Flinn IW, Ghia P, Eradat H, Ervin T, Lamanna N, Coiffier B, Pettitt AR, Ma S, Tausch E, Cramer P, Huang J, Mitra S, Hallek M, OBrien SM, Stilgenbauer S, (2019) Final Results of a Randomized, Phase III Study of Rituximab With or Without Idelalisib Followed by Open-Label Idelalisib in Patients with Relapsed Chronic Lymphocytic Leukemia. J Clin Oncol 37(16):1391–1402. https://doi.org/10.1200/JCO.18
Patnaik A, Appleman LJ, Tolcher AW, Papadopoulos KP, Beeram M, Rasco DW, Weiss GJ, Sachdev JC, Chadha M, Fulk M, Ejadi S, Mountz JM, Lotze MT, Toledo FG, Chu E, Jeffers M, Pena C, Xia C, Reif S, Genvresse I, Ramanathan RK (2016) First-in-human phase I study of copanlisib (BAY 80–6946), an intravenous pan-class I phosphatidylinositol 3-kinase inhibitor, in patients with advanced solid tumors and non-Hodgkin's lymphomas. Ann Oncol 27(10):1928–1940. https://doi.org/10.1093/annonc/mdw282
Dreyling M, Santoro A, Mollica L, Leppa S, Follows GA, Lenz G, Kim WS, Nagler A, Panayiotidis P, Demeter J, Ozcan M, Kosinova M, Bouabdallah K, Sieidel H, Pena C, Yin S, Hiemeyer F, Garcia-Vargas J, Childs BH, Zinzani PL (2017) Phosphatidylinositol 3-kinase inhibition by copanlisib in relapsed or refractory indolent lymphoma. J Clin Oncol 35(35):3898–3905. https://doi.org/10.1200/JCO.2017.75.4648
Horwitz SM, Koch R, Porcu P, Oki Y, Moskowitz A, Perez M, Myskowski P, Officer A, Jaffe JD, Morrow SN, Allen K, Douglas M, Stern H, Sweeney J, Kelly P, Kelly V, Aster JC, Weaver D, Foss FM, Weinstock DM (2018) Activity of the PI3K-delta-gamma inhibitor duvelisib in a phase 1 trial and preclinical models of T-cell lymphoma. Blood 131(8):888–898
Flinn IW, Hillmen P, Montillo M, Nagy Z, Illes A, Etienne G, Delgado J, Kuss BJ, Tam CS, Gasztonyi Z, Offner F, Lunin S, Bosch F, Davids MS, Lamanna N, Jaeger U, Ghia P, Cymbalista F, Portell CA, Skarbnik AP, Cashen AF, Weaver DT, Kelly VM, Turnbull B, Stilgenbauer S (2018) The phase 3 Duo trial: duvelisib vs ofatumumab in relapsed and refractory CLL/SLL. Blood 132(23):2446–2455
Flinn IW, Kahl B, Patel M, Oki Y, Foss FF, Porcu P, Jones J, Burger JA, Jain N, Kelly VM, Allen K, Douglas M, Sweeney J, Kelly P, Horwitz S (2018) Duvelisib, a novel oral dual inhibitor of PI3K-delta, gamma, is clinically active in advanced hematologic malignancies. Blood 131(8):877–887
Flinn IW, Montillo M, Nagy Z, Illes A, Etienne G, Delgado J, Kuss BJ, Tam CS, Gasztonyi Z, Offner F, Lunin S, Bosch F, Davids MS, Lamanna N, Jaeger U, Ghia P, Cymbalista F, Portell CA, Skarbnik AP, Cashen AF, Weaver DT, Kelly VM, Stilgenbauer S (2018) The Phase 3 DUO trial: duvelisib vs ofatumumab in relapsed and refractory CLL/SLL. Blood 132(23):2446–2455
Fruman DA, Chiu H, Hopkins BD, Bagrodia S, Cantley LC, Abraham RT (2017) The PI3K pathway in human disease. Cell 170(4):605–635. https://doi.org/10.1016/j.cell.2017.07.029
Hopkins BD, Pauli C, Du X, Wang DG, Li X, Wu D, Amadiume SC, Goncalves MD, Hodakoski C, Lundquist MR, Bareja R, Ma Y, Harris EM, Sboner A, Beltran H, Rubin MA, Mukherjee S, Cantley LC (2018) Suppression of insulin feedback enhances the efficacy of PI3K inhibitors. Nature 560(7719):499–503. https://doi.org/10.1038/s41586-018-0343-4
Podsypanina K, Ellenson LH, Nemes A, Gu J, Tamura M, Yamada KM, Cordon-Cardo C, Catoretti G, Fisher PE, Parsons R (1999) Mutations of Pten/Mmac1 in mice causes neoplasia in multiple organ systems. PNAS 96:1563–1568
Ying Z, Sandoval M, Beronja S (2018) Oncogenic activation of PI3K induces progenitor cell differentiation to suppress epidermal growth. Nat Cell Biol 20(11):1256–1266. https://doi.org/10.1038/s41556-018-0218-9
Calautti E, Li J, Saoncella S, Brissette JL, Goetinck PF (2005) Phosphoinositide 3-kinase signaling to Akt promotes keratinocyte differentiation versus death. J Biol Chem 280(38):32856–32865. https://doi.org/10.1074/jbc.M506119200
Stevenson S, Thronton J (2007) Effect of estrogens on skin aging and the potential of SERMs. Clin Interv Aging 2(3):283–297
Juric D, de Bono JS, LoRusso PM, Nemunaitis J, Heath EI, Kwak EL, Macarulla Mercade T, Geuna E, Jose de Miguel-Luken M, Patel C, Kuida K, Sankoh S, Westin EH, Zohren F, Shou Y, Tabernero J (2017) A first-in-human, phase I, dose-escalation study of TAK-117, a selective PI3Kalpha Isoform Inhibitor, in patients with advanced solid malignancies. Clin Cancer Res 23(17):5015–5023. https://doi.org/10.1158/1078-0432.CCR-16-2888
Hong R, Edgar K, Song K, Steven S, Young A, Hamilton P, Arrazate A, De La Cruz C, Chan C, Pang J, Salphati L, Belvin M, Nannini M, Staben S, Friedman L, Sampath D (2018) Abstract PD4-14: GDC-0077 is a selective PI3Kalpha inhibitor that demonstrates robust efficacy in PIK3CA mutant breast cancer models as a single agent and in combination with standard of care therapies. 2017 San Antonio Breast Cancer Symposium; December 5-9, 2017; San Antonio, Texas
Thangam EB, Jemima EA, Singh H, Baig MS, Khan M, Mathias CB, Church MK, Saluja R (2018) The role of histamine and histamine receptors in mast cell-mediated allergy and inflammation: the hunt for new therapeutic targets. Front Immunol 9:1873. https://doi.org/10.3389/fimmu.2018.01873
Togias A (2003) H1-receptors: Localization and role in airway physiology and in immune functions. J Allergy Clin Immunol 112(4):S60–S68. https://doi.org/10.1016/s0091-6749(03)01878-5
Acknowledgements
The authors thank the patients and staff at Memorial Sloan Kettering Cancer Center.
Funding
This work was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748. DGW is supported by a Medical Scientist Training Program grant from the NIH (T32GM007739) to the Weill Cornell/Rockefeller/Sloan-Kettering Tri-Institutional MD-PhD Program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
DGW declares no conflicts of interest. DMB declares no conflicts of interest. VSB declares consulting/advisory agreements with Pfizer and Anthem Foundation. VSB receives research funding from the NIH (5 R37 CA214785). JFB declares no conflicts of interest. PRD declares no conflicts of interest. SAF receives research funding from Genentech/Roche, AstraZeneca, and Decibel Therapeutics. SAF has stock/equity ownership in Urogen Pharma, Allogene Therapeutics, Kronos Bio, Vida Ventures, Kite Pharma, and Neogene Therapeutics. KLJ declares consulting/advisory board roles with Novartis, Spectrum Pharmaceuticals, ADC Therapeutics, Pfizer, BMS, Abbvie, AstraZeneca, Jounce Therapeutics, Taiho, Oncology, Genentech, Synthon, Lilly Pharmaceuticals, and Intellisphere. KLJ receives research funding from Novartis, Clovis Oncology, Genentech, Astra Zeneca, ADC Therapeutics, Novita Pharmaceuticals, Debio Pharmaceuticals, Pfizer, Lilly Pharmaceuticals, Zymeworks, Immunomedics, and Puma Biotechnology. DEL declares no conflicts of interest. TL declares no conflicts of interest. SM declares consulting/advisory agreements with Daiichi Sankyo, Carrick, Eli Lilly, Genentech, MacroGenics, and GSK Speakers Bureau Genetech. SM receives research support from Novartis, Genentech, Astra Zeneca/ MedImmune, Seattle Genetics, and Daiichi Sankyo. PR declares a consulting/advisory agreement with Novartis. PR receives institutional research support from Illumina and GRAIL. MS is an employee of Novartis. TAT declares consulting/advisory agreements with Genentech/Roche, Pfizer, AstraZeneca, Merck, Puma, Advaxis, Celgene, Innocrin, Genomic Health, Bristol-Myers Squibb, Samsung, Athenex, Aduro Biotech, Halozyme, Daiichi Sankyo, Ionis. TT receives research funding from Eisai, Pfizer, Novartis, Innocrin, AstraZeneca, Astellas, Immunomedics, Genentech, Daiichi, and Carrick. LTV declares no conflicts of interest. MEL declares a consulting/advisory agreement with Novartis.
Ethical approval
Our retrospective research study was approved by and in accordance with the ethical standards of Memorial Sloan Kettering Cancer Center’s Institutional Review Board committee (Protocol # 16-458). This article does not contain any studies with human or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, D.G., Barrios, D.M., Blinder, V.S. et al. Dermatologic adverse events related to the PI3Kα inhibitor alpelisib (BYL719) in patients with breast cancer. Breast Cancer Res Treat 183, 227–237 (2020). https://doi.org/10.1007/s10549-020-05726-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-020-05726-y