Abstract
Purpose
Treatment with Palbociclib, a cyclin-dependent kinase 4/6 inhibitor, has demonstrated significantly improved progression-free survival in patients with hormone receptor-positive, HER2-negative, advanced breast cancer, when used in combination with letrozole or fulvestrant endocrine therapies. However, limited information exists on its cutaneous adverse effects (AE). Hence, we conducted a retrospective cohort study to investigate the prevalence and management of cutaneous AE during palbociclib and endocrine therapy.
Method
We included 324 adult patients with advanced breast cancer who received palbociclib between March 2016 and August 2020 within a tertiary comprehensive cancer centre. Patient demographics, details of previous and concurrent treatments, as well as treatment-related cutaneous AE were recorded from electronic records.
Results
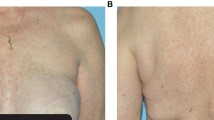
The incidence of treatment-related cutaneous AE was 14.2% (46 from a total of 324 patients). The most frequent cutaneous reactions included maculopapular rash (41%), asteatosis (37%), pruritus and urticaria (20%), and bullous dermatitis reactions (9%). We identified two patients with treatment-induced subacute cutaneous lupus erythematosus, one case of bullous pemphigoid, and a single erythema multiforme. Patients received an average of 9 cycles of treatment, completing an average of 6 cycles before developing cutaneous AE, which persisted for a median of 43 days. Only 15% (n = 7) of affected patients required temporary suspension, and 4% (n = 2) required discontinuation. The majority were managed with potent topical steroids, with oral corticosteroids being required in 3 patients, and one patient required hydroxychloroquine.
Conclusion
Our study describes both the spectrum of cutaneous AE of palbociclib and endocrine therapy, and approaches to management. Prompt management may limit the negative impact on patients, facilitating beneficial continuation of palbociclib and endocrine therapy.







Similar content being viewed by others
References
Cancer Research UK, https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/breast-cancer/incidence-invasive, Accessed [September 2019]
Finn RS, Crown JP, Lang I, Boer K, Bondarenko IM, Kulyk SO et al (2015) The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol 16(1):25–35
Finn RS, Martin M, Rugo HS, Jones S, Im S-A, Gelmon K et al (2016) Palbociclib and letrozole in advanced breast cancer. N Engl J Med 375(20):1925–1936
Turner NC, Slamon DJ, Ro J, Bondarenko I, Im S-A, Masuda N et al (2018) Overall survival with palbociclib and fulvestrant in advanced breast cancer. N Engl J Med 379(20):1926–1936
Turner NC, Ro J, André F, Loi S, Verma S, Iwata H et al (2015) Palbociclib in hormone-receptor–positive advanced breast cancer. N Engl J Med 373(3):209–219
CTCAE criteria: National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) v.4 data files. Available at: http://evs.nci.nih.gov/ftp1/CTCAE/About.html [Accessed January 2020]
Pascual J, MacPherson IR, Armstrong AC, Ward SE, Parmar M, Turner AJ et al (2019) PIPA: A phase Ib study of β-isoform sparing phosphatidylinositol 3-kinase (PI3K) inhibitor taselisib (T) plus palbociclib (P) and fulvestrant (FUL) in PIK3CA-mutant (mt) ER-positive and taselisib (T) plus palbociclib (P) in PIK3CA-mutant (mt) ER-negative advanced breast cancer. JCO. 37(15):1051–1051
Sibaud V (2018) Dermatologic reactions to immune checkpoint inhibitors. Am J Clin Dermatol 19(3):345–361
Damsky W, Kole L, Tomayko MM (2016) Development of bullous pemphigoid during nivolumab therapy. JAAD Case Rep 2:442–444
Stavropoulos PG, Soura E, Antoniou C (2014) Drug-induced pemphigoid: a review of the literature. J Eur Acad Dermatol Venereol 28(9):1133–1140
Kanahara SM, Agrawal A (2016) Drug-induced bullous pemphigoid. J Gen Intern Med 31(11):1393–1394. https://doi.org/10.1007/s11606-016-3679-1
Chung VQ, Moschella SL, Zembowicz A, Liu V (2006) Clinical and pathologic findings of paraneoplastic dermatoses. J Am Acad Dermatol 54:745
Wiznia LE, Subtil A, Choi JN (2013) Subacute cutaneous lupus erythematosus induced by chemotherapy: gemcitabine as a causative agent. JAMA Dermatol 149(9):1071–1075
Cleaver N, Ramirez J, Gildenberg S (2013) Cutaneous lupus erythematosus in a patient undergoing intravitreal bevacizumab injections: case report and review of the literature. J Drugs Dermatol 12(9):1052–1055
Pinard J, Patel M, Granter SR, Vleugels RA, Merola JF (2018) Subacute cutaneous lupus erythematosus induced by palbociclib. J Cutan Med Surg 22(3):341–343
Freedman JB, Herskovitz I, Maderal AD (2019) Chronic cutaneous lupus erythematosus (discoid lupus) induced by palbociclib. Int J Dermatol. https://doi.org/10.1111/ijd.14716
Calabrese G, Licata G, Gambardella A, De Rosa A, Ronchi A, Argenziano G (2020) A case of discoid lupus erythematosus because of palbociclib. J Cutan Pathol. https://doi.org/10.1111/cup.13696
Wilkerson E, Hazey MA, Bahrami S, Callen JP (2012) Golimumab-Exacerbated Subacute Cutaneous Lupus Erythematosus. Arch Dermatol 148(10):1186–1190
Furukawa F (1999) Antinuclear antibody-keratinocyte interactions in photosensitive cutaneous lupus erythematosus. Histol Histopathol 14(2):627–633
Karagounis T, Vallurupalli M, Nathan N et al (2018) Stevens-Johnson syndrome-like eruption from palbociclib in a patient with metastatic breast cancer. JAAD Case Rep 4(5):452–454
Widmer S, Grossman M (2018) Chemotherapy patient with stevens-johnson syndrome presents to the emergency department: a case report. Am J Emerg Med 36(7):1325.e3-1325.e4
Santoro S, Santini M, Pepe C, Tognetti E, Cortelazzi C, Ficarelli E, De Panfilis G (2011) Aromatase inhibitor-induced skin adverse reactions: exemestane-related cutaneous vasculitis. J Eur Acad Dermatol Venereol 25:596–598
Abdou NI, Rider V, Greenwell C, Li X, Kimler BF (2008) Fulvestrant (Faslodex), an estrogen selective receptor downregulator, in therapy of women with systemic lupus erythematosus clinical, serologic, bone density, and T cell activation marker studies: a double-blind placebo-controlled trial. J Rheumatol. 35(5):797
Funding
There are no funding sources to declare.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest to disclose.
Informed consent
The patients in this manuscript have given written informed consent to publication of their case details.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chawla, S., Hill, A., Fearfield, L. et al. Cutaneous toxicities occurring during palbociclib (CDK4/6 inhibitor) and endocrine therapy in patients with advanced breast cancer: a single-centre experience. Breast Cancer Res Treat 188, 535–545 (2021). https://doi.org/10.1007/s10549-021-06169-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-021-06169-9