Abstract
Purpose
Limited evidence exists on the impact of hormone receptor (HR) status to counsel HER2-positive early breast cancer patients receiving adjuvant anti-HER2 therapy.
Methods
ALTTO (BIG 2-06) was an international, intergroup, open-label, randomized phase III trial in HER2-positive early breast cancer patients randomized to receive 1 year of trastuzumab and/or lapatinib. HER2, estrogen and progesterone receptors were centrally tested for all patients. We investigated the impact of HR status on prognosis, risk of disease-free survival (DFS) events over time, patterns of first DFS events, and factors associated with risk of DFS events overall, in years 0–5 and 6–8.
Results
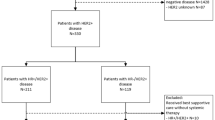
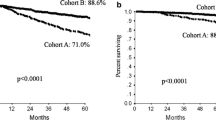
Out of 6273 patients included in this analysis, 3603 (57.4%) had HR-positive tumors. Median follow-up was 6.93 years. Five-year and 8-year DFS were 86% and 80% in patients with HR-positive disease, and 83% and 79% in those with HR-negative tumors, respectively. Mean annual hazards of recurrence in years 0–5 were 3% in patients with HR-positive disease and 4% in those with HR-negative tumors, while in years 6–8 they were 3% and 2%, respectively. Distribution of first DFS event in years 6–8 (P = 0.005) and type of first distant recurrence (P < 0.001) were significantly different between the two groups. Risk factors for DFS events overall, in years 0–5, and 6–8 were different in patients with HR-positive and HR-negative tumors.
Conclusions
HER2-positive early breast cancer is characterized by the presence of two diseases with distinct natural history based on HR status requiring the development of different follow-up strategies and future de-escalation and escalation clinical trials.


Similar content being viewed by others
References
Nicolini A, Ferrari P, Duffy MJ (2018) Prognostic and predictive biomarkers in breast cancer: past, present and future. Semin Cancer Biol 52(Pt 1):56–73
Vaz-Luis I, Winer EP, Lin NU (2013) Human epidermal growth factor receptor-2-positive breast cancer: does estrogen receptor status define two distinct subtypes? Ann Oncol 24(2):283–291
Schettini F, Buono G, Cardalesi C, Desideri I, De Placido S, Del Mastro L (2016) Hormone receptor/human epidermal growth factor receptor 2-positive breast cancer: where we are now and where we are going. Cancer Treat Rev 46:20–26
Prat A, Perou CM (2011) Deconstructing the molecular portraits of breast cancer. Mol Oncol. 5(1):5–23
Cheang MCU, Martin M, Nielsen TO, Prat A, Voduc D, Rodriguez-Lescure A et al (2015) Defining breast cancer intrinsic subtypes by quantitative receptor expression. Oncologist 20(5):474–482
Ferrari A, Vincent-Salomon A, Pivot X, Sertier A-S, Thomas E, Tonon L et al (2016) A whole-genome sequence and transcriptome perspective on HER2-positive breast cancers. Nat Commun. 7:12222
Untch M, Gelber RD, Jackisch C, Procter M, Baselga J, Bell R et al (2008) Estimating the magnitude of trastuzumab effects within patient subgroups in the HERA trial. Ann Oncol 19(6):1090–1096
Strasser-Weippl K, Horick N, Smith IE, O’Shaughnessy J, Ejlertsen B, Boyle F et al (2015) Long-term hazard of recurrence in HER2+ breast cancer patients untreated with anti-HER2 therapy. Breast Cancer Res 17:56
Loi S, Dafni U, Karlis D, Polydoropoulou V, Young BM, Willis S et al (2016) Effects of estrogen receptor and human epidermal growth factor receptor-2 levels on the efficacy of trastuzumab: a secondary analysis of the HERA trial. JAMA Oncol. 2(8):1040–1047
Curigliano G, Burstein HJ, Winer PE, Gnant M, Dubsky P, Loibl S et al (2017) De-escalating and escalating treatments for early-stage breast cancer: the Gallen international expert consensus conference on the primary therapy of early breast cancer 2017. Ann Oncol 28(8):1700–1712
Denduluri N, Chavez-MacGregor M, Telli ML, Eisen A, Graff SL, Hassett MJ et al (2018) Selection of optimal adjuvant chemotherapy and targeted therapy for early breast cancer: ASCO clinical practice guideline focused update. J Clin Oncol 36(23):2433–2443
Piccart-Gebhart M, Holmes E, Baselga J, de Azambuja E, Dueck AC, Viale G et al (2016) Adjuvant lapatinib and trastuzumab for early human epidermal growth factor receptor 2-positive breast cancer: results from the randomized phase iii adjuvant lapatinib and/or trastuzumab treatment optimization trial. J Clin Oncol 34(10):1034–1042
Moreno-Aspitia A, Holmes EM, Jackisch C, De Azambuja E, Boyle FM, Hillman DW, et al. Updated results from the phase III ALTTO trial (BIG 2-06; NCCTG (Alliance) N063D) comparing one year of anti-HER2 therapy with lapatinib alone (L), trastuzumab alone (T), their sequence (T → L) or their combination (L + T) in the adjuvant treatment of HER2-positive early breast cancer. J Clin Oncol. 2017;35(suppl; abstr 502)
Wolff AC, Hammond MEH, Schwartz JN, Hagerty KL, Allred DC, Cote RJ et al (2007) American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol 25(1):118–145
Metzger-Filho O, Sun Z, Viale G, Price KN, Crivellari D, Snyder RD et al (2013) Patterns of Recurrence and outcome according to breast cancer subtypes in lymph node-negative disease: results from international breast cancer study group trials VIII and IX. J Clin Oncol 31(25):3083–3090
Cossetti RJD, Tyldesley SK, Speers CH, Zheng Y, Gelmon KA (2015) Comparison of breast cancer recurrence and outcome patterns between patients treated from 1986 to 1992 and from 2004 to 2008. J Clin Oncol 33(1):65–73
von Minckwitz G, Procter M, de Azambuja E, Zardavas D, Benyunes M, Viale G et al (2017) Adjuvant pertuzumab and trastuzumab in early HER2-Positive breast cancer. N Engl J Med 377(2):122–131
Martin M, Holmes FA, Ejlertsen B, Delaloge S, Moy B, Iwata H et al (2017) Neratinib after trastuzumab-based adjuvant therapy in HER2-positive breast cancer (ExteNET): 5-year analysis of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 18(12):1688–1700
Giuliano M, Hu H, Wang Y-C, Fu X, Nardone A, Herrera S et al (2015) Upregulation of ER signaling as an adaptive mechanism of cell survival in HER2-positive breast tumors treated with anti-HER2 therapy. Clin Cancer Res 21(17):3995–4003
Park YH, Lee S, Cho EY, Choi YL, Lee JE, Nam SJ et al (2010) Patterns of relapse and metastatic spread in HER2-overexpressing breast cancer according to estrogen receptor status. Cancer Chemother Pharmacol 66(3):507–516
Kennecke H, Yerushalmi R, Woods R, Cheang MCU, Voduc D, Speers CH et al (2010) Metastatic behavior of breast cancer subtypes. J Clin Oncol 28(20):3271–3277
Vaz-Luis I, Ottesen RA, Hughes ME, Marcom PK, Moy B, Rugo HS et al (2012) Impact of hormone receptor status on patterns of recurrence and clinical outcomes among patients with human epidermal growth factor-2-positive breast cancer in the National Comprehensive Cancer Network: a prospective cohort study. Breast Cancer Res 14(5):R129
Hess KR, Esteva FJ (2013) Effect of HER2 status on distant recurrence in early stage breast cancer. Breast Cancer Res Treat 137(2):449–455
Kang Y, Siegel PM, Shu W, Drobnjak M, Kakonen SM, Cordón-Cardo C et al (2003) A multigenic program mediating breast cancer metastasis to bone. Cancer Cell 3(6):537–549
Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu W, Giri DD et al (2005) Genes that mediate breast cancer metastasis to lung. Nature 436(7050):518–524
Bos PD, Zhang XHF, Nadal C, Shu W, Gomis RR, Nguyen DX et al (2009) Genes that mediate breast cancer metastasis to the brain. Nature. 459(7249):1005–1009
Klein A, Olendrowitz C, Schmutzler R, Hampl J, Schlag PM, Maass N et al (2009) Identification of brain- and bone-specific breast cancer metastasis genes. Cancer Lett 276(2):212–220
Moschetti I, Cinquini M, Lambertini M, Levaggi A, Liberati A (2016) Follow-up strategies for women treated for early breast cancer. Cochrane Database Syst Rev. 5:CD001768
Dood RL, Zhao Y, Armbruster SD, Coleman RL, Tworoger S, Sood AK et al (2018) Defining survivorship trajectories across patients with solid tumors: an evidence-based approach. JAMA Oncol. 4(11):1519–1526
Yi M, Huo L, Koenig KB, Mittendorf EA, Meric-Bernstam F, Kuerer HM et al (2014) Which threshold for ER positivity? a retrospective study based on 9639 patients. Ann Oncol 25(5):1004–1011
Ellis MJ, Coop A, Singh B, Tao Y, Llombart-Cussac A, Jänicke F et al (2003) Letrozole inhibits tumor proliferation more effectively than tamoxifen independent of HER1/2 expression status. Cancer Res 63(19):6523–6531
Francis PA, Pagani O, Fleming GF, Walley BA, Colleoni M, Láng I et al (2018) Tailoring adjuvant endocrine therapy for premenopausal breast cancer. N Engl J Med 379(2):122–137
Bartlett JMS, Ahmed I, Regan MM, Sestak I, Mallon EA, Dell’Orto P et al (2017) HER2 status predicts for upfront AI benefit: a TRANS-AIOG meta-analysis of 12,129 patients from ATAC, BIG 1-98 and TEAM with centrally determined HER2. Eur J Cancer 79:129–138
Biganzoli E, Desmedt C, Fornili M, de Azambuja E, Cornez N, Ries F et al (2017) Recurrence dynamics of breast cancer according to baseline body mass index. Eur J Cancer 87:10–20
Hayes DF, Thor AD, Dressler LG, Weaver D, Edgerton S, Cowan D et al (2007) HER2 and response to paclitaxel in node-positive breast cancer. N Engl J Med 357(15):1496–1506
Acknowledgements
Matteo Lambertini acknowledges the support from the European Society for Medical Oncology (ESMO) for a Translational Research Fellowship at the Institut Jules Bordet in Brussels (Belgium) during the conduction of this analysis. We also acknowledge the ALTTO staff of the BrEAST Data Centre at Institut Jules Bordet in Brussels (Belgium), for clinical record online management, and Sebastien Guillaume of BrEAST Data Centre at Institut Jules Bordet in Brussels (Belgium) for administrative support. None of the individuals named in the acknowledgment received any compensation for their contributions.
Funding
The ALTTO trial received financial support from GlaxoSmithKline (until January 2015), Novartis Pharma AG (as of January 2015), and the National Cancer Institute of the National Institutes of Health (NCI-NIH; Grant No. U10CA180821 and U10CA180882 to the Alliance for Clinical Trials in Oncology and Grant No. CA025224 to the legacy North Central Cancer Treatment Group). The present analysis did not receive additional funding. The funders and sponsors had no role in the design or conduct of the study, in the collection, analysis, or interpretation of the data, neither in the preparation, review, or approval of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Lambertini received honoraria from Teva and speaker’s bureau from Theramex outside the submitted work. Dr. Gelber received research grants from Novartis Pharma AG, Pfizer, Roche-Genentech, Ipsen, Celgene, Merck, and Ferring (to the institution). Dr. Werner reports employment at Novartis Pharma AG. Dr. Vaz-Luis received honoraria from Novartis Pharma AG, AstraZeneca, and Ipsen/Kephren outside the submitted work, and research grants from Susan Komen for Cure and Odyssea. Dr. Piccart is a board member of Radius, received honoraria from AstraZeneca, Lilly, MSD, Novartis Pharma AG, Odonate, Pfizer, Roche-Genentech, Camel-IDS, Crescendo Biologics, Periphagen, Huya, Debiopharm, PharmaMar, G1 Therapeutics, Menarini, Seattle Genetics, Immunomedics, Oncolytics outside the submitted work, and research grants from AstraZeneca, Lilly, MSD, Novartis Pharma AG, Pfizer, Roche-Genentech, Synthon, Radius, Servier (to the institution). Dr. Loi acted as consultant (not compensated) to Seattle Genetics, Pfizer, Novartis Pharma AG, BMS, Merck, and Roche-Genentech outside the submitted work, and received research grants from Novartis Pharma AG, Bristol Meyers Squibb, Merck, Roche-Genentech, Puma Biotechnology, and Pfizer (to the institution). Dr. de Azambuja received honoraria from Roche-Genentech, research grants from Roche–Genentech (to the institution), and travel grants from Roche-Genentech and GlaxoSmithKline outside the submitted work. All the other authors declare no competing interests.
Ethical approval
All procedures performed in this trial involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lambertini, M., Campbell, C., Gelber, R.D. et al. Dissecting the effect of hormone receptor status in patients with HER2-positive early breast cancer: exploratory analysis from the ALTTO (BIG 2-06) randomized clinical trial. Breast Cancer Res Treat 177, 103–114 (2019). https://doi.org/10.1007/s10549-019-05284-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-019-05284-y