Abstract
Purpose
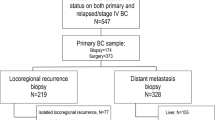
HER2 expression has been reported to be discordant between primary tumor and metastatic tissue.
Patients and methods
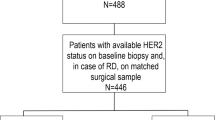
HER2 discordance and relation to HER2-targeted treatment was investigated in 227 patients with primary breast cancer.
Results
HER2 discordance between primary biopsy and second biopsy after neoadjuvant or adjuvant treatment was observed in 20.7%. This discordance was related only to the use of HER2-targeted treatment: 30 of 33 (90.9%) women with downgraded HER2 expression underwent a HER2-targeted therapy, whereas in the group of patients with concordant HER2 expression, only 32 of 180 (17.8%) received HER2-targeted treatment (p < 0.0001). HER2 discordance was associated with reduced disease-free survival but not overall survival. In a second cohort, including patients with HER2 overexpressing tumors, trastuzumab treatment was associated with change of HER2 expression from positive to negative in 47.3% of cases. Addition of pertuzumab increased the rate of HER2 loss up to 63.2%. Notably, the interval between last HER2-targeted treatment and the time of surgical excision of the tumor after neoadjuvant chemotherapy (NACT) or the biopsy of the metachronous metastasis was associated with a significant change in HER2 expression. The median time between NACT and the time of surgical excision was 23 days (range 5–81 days) for tumors with decreased HER2 expression and 51 days (range 10–179 days) for tumors with concordant HER2 expression. Furthermore, median time between the end of adjuvant treatment and second histology of the metachronous metastases accounted for 15 days (range 2–165 days) and 478 days (range 7–2739 days) was observed in the group of patients with decreased or unchanged HER2 expression, respectively.
Conclusion
The interval between anti-HER2 treatment and the determination of HER2 in second histology is strongly associated with HER2 expression.



Similar content being viewed by others
References
Ignatov T, Eggemann H, Burger E, Costa SD, Ignatov A (2016) Hormone receptor status does not alter the effect of trastuzumab in breast cancer. Endocr Relat Cancer 23:349–355
Niikura N, Tomotaki A, Miyata H et al (2016) Changes in tumor expression of HER2 and hormone receptors status after neoadjuvant chemotherapy in 21,755 patients from the Japanese breast cancer registry. Ann Oncol 27:480–487
Gahlaut R, Bennett A, Fatayer H et al (2016) Effect of neoadjuvant chemotherapy on breast cancer phenotype, ER/PR and HER2 expression - Implications for the practising oncologist. Eur J Cancer 60:40–48
Yeung C, Hilton J, Clemons M et al (2016) Estrogen, progesterone, and HER2/neu receptor discordance between primary and metastatic breast tumours-a review. Cancer Metastasis Rev 35:427–437
van de Ven S, Smit VT, Dekker TJ, Nortier JW, Kroep JR (2011) Discordances in ER, PR and HER2 receptors after neoadjuvant chemotherapy in breast cancer. Cancer Treat Rev 37:422–430
Mittendorf EA, Wu Y, Scaltriti M et al (2009) Loss of HER2 amplification following trastuzumab-based neoadjuvant systemic therapy and survival outcomes. Clin Cancer Res 15:7381–7388
Nakamura R, Yamamoto N, Shiina N et al (2016) Impact of host and histopathological factors on the discrepancies in estrogen receptor, and progesterone receptor, and HER2 status between core needle biopsy and surgically excised tumors. Breast 26:141–147
Bottini A, Berruti A, Bersiga A et al (1996) Effect of neoadjuvant chemotherapy on Ki67 labelling index, c-erbB-2 expression and steroid hormone receptor status in human breast tumours. Anticancer Res 16:3105–3110
Vincent-Salomon A, Jouve M, Genin P et al (2002) HER2 status in patients with breast carcinoma is not modified selectively by preoperative chemotherapy and is stable during the metastatic process. Cancer 94:2169–2173
Eggemann H, Ignatov T, Burger E et al (2015) Moderate HER2 expression as a prognostic factor in hormone receptor positive breast cancer. Endocr Relat Cancer 22:725–733
Ignatov T, Eggemann H, Burger E, Fettke F, Costa SD, Ignatov A (2015) Moderate level of HER2 expression and its prognostic significance in breast cancer with intermediate grade. Breast Cancer Res Treat 151:357–364
Wolff AC, Hammond ME, Hicks DG et al (2013) Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol 31:3997–4013
von Elm E, Altman DG, Egger M et al (2007) The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 370:1453–1457
Niikura N, Liu J, Hayashi N et al (2012) Loss of human epidermal growth factor receptor 2 (HER2) expression in metastatic sites of HER2-overexpressing primary breast tumors. J Clin Oncol 30:593–599
Guarneri V, Dieci MV, Barbieri E et al (2013) Loss of HER2 positivity and prognosis after neoadjuvant therapy in HER2-positive breast cancer patients. Ann Oncol 24:2990–2994
Cuello M, Ettenberg SA, Clark AS et al (2001) Down-regulation of the erbB-2 receptor by trastuzumab (herceptin) enhances tumor necrosis factor-related apoptosis-inducing ligand-mediated apoptosis in breast and ovarian cancer cell lines that overexpress erbB-2. Cancer Res 61:4892–4900
Baselga J, Albanell J, Molina MA, Arribas J (2001) Mechanism of action of trastuzumab and scientific update. Semin Oncol 28:4–11
Hughes JB, Rodland MS, Hasmann M, Madshus IH, Stang E (2012) Pertuzumab increases 17-AAG-induced degradation of ErbB2, and this effect is further increased by combining pertuzumab with trastuzumab. Pharmaceuticals (Basel) 5:674–689
Nahta R, Hung MC, Esteva FJ (2004) The HER-2-targeting antibodies trastuzumab and pertuzumab synergistically inhibit the survival of breast cancer cells. Cancer Res 64:2343–2346
Longva KE, Pedersen NM, Haslekas C, Stang E, Madshus IH (2005) Herceptin-induced inhibition of ErbB2 signaling involves reduced phosphorylation of Akt but not endocytic down-regulation of ErbB2. Int J Cancer 116:359–367
Bertelsen V, Stang E (2014) The mysterious ways of ErbB2/HER2 trafficking. Membranes (Basel) 4:424–446
Press MF, Sauter G, Bernstein L et al (2005) Diagnostic evaluation of HER-2 as a molecular target: an assessment of accuracy and reproducibility of laboratory testing in large, prospective, randomized clinical trials. Clin Cancer Res 11:6598–6607
Lee HJ, Kim JY, Park SY et al (2015) Clinicopathologic Significance of the Intratumoral Heterogeneity of HER2 Gene Amplification in HER2-Positive Breast Cancer Patients Treated With Adjuvant Trastuzumab. Am J Clin Pathol 144:570–578
Saeki H, Oki E, Kashiwada T et al (2018) Re-evaluation of HER2 status in patients with HER2-positive advanced or recurrent gastric cancer refractory to trastuzumab (KSCC1604). Eur J Cancer 105:41–49
Funding
This research did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest.
Ethical approval
The experiments comply with the current laws of Germany and were performed according to the good clinical practice (GCP) guidelines.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ignatov, T., Gorbunow, F., Eggemann, H. et al. Loss of HER2 after HER2-targeted treatment. Breast Cancer Res Treat 175, 401–408 (2019). https://doi.org/10.1007/s10549-019-05173-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-019-05173-4