Abstract
Approximately a half of breast tumors classified as HER2-negative exhibit HER2-low-positive expression. We recently described a high instability of HER2-low-positive expression from primary breast cancer (BC) to relapse. Previous studies reporting discordance in HER2 status between baseline biopsy and residual disease (RD) in patients undergoing neoadjuvant treatment did not include the HER2-low-positive category. The aim of this study is to track the evolution of HER2-low-positive expression from primary BC to RD after neoadjuvant treatment. Patients undergoing neoadjuvant treatment with available baseline tumor tissue and matched samples of RD (in case of no pCR) were included. HER2-negative cases were sub-classified as HER2-0 or HER2-low-positive (IHC 1+ or 2+ and ISH negative). Four-hundred forty-six patients were included. Primary BC phenotype was: HR-positive/HER2-negative 23.5%, triple-negative (TN) 35%, HER2-positive 41.5%. HER2-low-positive cases were 55.6% of the HER2-negative cohort and were significantly enriched in the HR-positive/HER2-negative vs. TN subgroup (68.6% vs. 46.8%, p = 0.001 χ2 test). In all, 35.3% of non-pCR patients (n = 291) had a HER2-low-positive expression on RD. The overall rate of HER2 expression discordance was 26.4%, mostly driven by HER2-negative cases converting either from (14.8%) or to (8.9%) HER2-low-positive phenotype. Among HR-positive/HER2-negative patients with HER2-low-positive expression on RD, 32.0% and 57.1% had an estimated high risk of relapse according to the residual proliferative cancer burden and CPS-EG score, respectively. In conclusion, HER2-low-positive expression showed high instability from primary BC to RD after neoadjuvant treatment. HER2-low-positive expression on RD may guide personalized adjuvant treatment for high-risk patients in the context of clinical trials with novel anti-HER2 antibody-drug conjugates.
Similar content being viewed by others
Introduction
Breast cancer is recognized as a highly heterogeneous disease in terms of biological features, prognosis and treatment sensitivity. In the past decades access to anti-HER2 drugs has been driven by the dichotomy between HER2-positive and HER2-negative breast cancer established in the context of pivotal trials of trastuzumab, however recent findings have challenged this dogma. In particular, early-phase clinical trials reported promising anti-tumor activity of the anti-HER2 antibody-drug conjugates (ADC) Trastuzumab-Deruxtecan and Trastuzumab-Duocarmazine in patients traditionally classified as having HER2-negative breast cancer though exhibiting HER2-low-positive expression (IHC scores 1+ or 2+ in the absence of HER2 gene amplification by ISH)1,2. In addition, recently, a phase II study treating 48 heavily pre-treated hormone-receptor (HR)-positive-HER2-low-positive advanced breast cancer patients with the anti-HER2/HER3 ADC zenocutuzumab (in combination with the last endocrine agent on which the patients had previously progressed immediately before the study entry), reported promising preliminary results in terms of clinical activity and safety3. These findings provided the proof of principle that novel anti-HER2 ADCs may exploit, to be active, the mere presence of the target, rather than a proven oncogene addiction to HER2. Two phase III clinical trials randomizing HER2-low-positive metastatic breast cancer patients to receive either Trastuzumab-Deruxtecan or treatment of physician’s choice are currently ongoing and results are pending (NCT03734029—Destiny-Breast04 met its primary endpoint—data not presented yet, NCT04494425—Destiny-Breast06). In addition, several other diverse combinations of anti-HER2 agents plus endocrine therapy/CDK 4/6 inhibitors/immunotherapy/other targeted agents are currently being tested in early-phase clinical trials, thus emphasizing the fervent interest on this dawning breast cancer subtype4. On the same grounds, ongoing efforts have been directed towards uncovering whether HER2-low-positive tumors may retain possible unique traits5,6,7, however, so far, solid evidence supporting HER2-low-positive breast cancer as a distinct biological and/or clinical entity is lacking.
We recently reported a high instability of HER2-low-positive expression from primary breast cancer to relapse8, suggesting that a not negligible proportion of patients originally classified as HER2-0 becomes HER2-low-positive at recurrence thus potentially expanding their therapeutic options.
Neoadjuvant treatment currently represents a widely adopted strategy for patients with early breast cancer given the well-acknowledged benefits in terms of expansion of locoregional treatment options, in-vivo evaluation of treatment sensitivity and the possibility to tailor post-neoadjuvant approach based on the pathological response after neoadjuvant treatment9,10. In this particular context, FDA has recently endorsed to select high-risk patients to be enrolled in adjuvant escalation trials based on the presence of residual disease after neoadjuvant therapy11. Interestingly, in patients undergoing neoadjuvant treatment and failing to achieve a pCR, the finding of discordance in HR and HER2 status from baseline biopsy to residual disease has been reported as a relatively frequent phenomenon12,13,14,15,16. However, previous studies did not include the emerging HER2-low-positive category in their evaluation.
In the present work, we investigated the evolution of HER2 expression from primary breast cancer to matched samples of residual disease in a large cohort of breast cancer patients undergoing neoadjuvant chemotherapy.
Results
Patient cohorts and clinicopathologic features
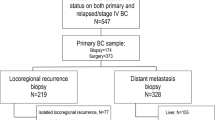
A total of 446 breast cancer patients undergoing neoadjuvant chemotherapy were included, as shown in Fig. 1. Table 1 shows main clinicopathologic features of the overall cohort. The majority of patients had tumors of ductal histology (n = 397, 89.0%) and poor differentiation (G3, n = 326, 70.9%) on baseline biopsy and almost 94% of patients had clinical stage II–III breast cancer (stage II: n = 259, 58,1%; stage III: n = 159, 35.7%). Tumor phenotypes on baseline biopsy were distributed as follows: HR-positive/HER2-negative 23.5% (n = 105), triple-negative 35% (n = 156) and HER2-positive 41.5% (n = 185; HER2-positive/HR-positive, n = 104; HER2-positive/HR-negative, n = 81). In the great majority of cases patients underwent anthracycline-taxane-based neoadjuvant chemotherapy (n = 354, 79.4%) and among HER2-positive patients, 86.5% received anti-HER2 blockade associated with neoadjuvant chemotherapy (n = 160). One-hundred fifty-five patients achieved pCR after neoadjuvant chemotherapy (34.8%). The distribution of pCR rates according to tumor phenotype is shown in Table 2. As expected, significantly higher rates of pCR were observed in triple-negative and HER2-positive subgroup as compared to HR-positive/HER2-negative subtype (38.5%, 45.9%, and 9.5% respectively, p < 0.001 χ2 test). Among 291 patients with residual disease after neoadjuvant therapy, the distribution of breast cancer phenotype was: HR-positive/HER2-negative 35.7% (n = 104), triple-negative 32% (n = 93) and HER2-positive 32.3% (n = 94; HER2-positive/HR-positive, n = 66; HER2-positive/HR-negative, n = 28). 17.9% of patients received further chemotherapy in the adjuvant setting (n = 80); among HER2-positive breast cancer patients, more than 90% received adjuvant anti-HER2 therapy (n = 177); 227 patients received adjuvant hormonal therapy.
Features of HER2-low-positive breast cancer in baseline biopsy and residual disease
Among HER2-negative cases (n = 261), the distribution of breast cancer phenotype on baseline biopsy according to HER2 expression was as follows: HER2-0 44.4% (n = 116), HER2-low-positive 55.6% (n = 145). A higher proportion of HER2-low-positive cases was observed in HR-positive/HER2-negative subgroup as compared to triple-negative subtype (68.6% vs. 46.8%, respectively, p = 0.001 χ2 test), as shown in Table 3.
In the subgroup of patients failing to achieve pCR (n = 291), the proportion of HER2-low-positive cases on residual disease was 35.3% (n = 103), corresponding to 52.3% of the HER2-negative cohort. Similar to the basal tissue samples, a highly significant association between HER2-low-positive expression on residual disease and HR status was observed (HER2-low-positive proportion among HR-positive and triple-negative cases: 65.4% vs. 37.6%, respectively, p < 0.001 χ2 test).
HER2 expression distribution according to tumor phenotype on RD in the HER2-negative cohort is shown in Table 3.
A significant association was observed between HER2 expression and the probability to achieve pCR. In particular, HER2-low-positive breast cancer was associated with the lowest rate of pCR, followed by HER2-0 and HER2-positive tumors (pCR rates HER2-low-positive vs. HER2-0 vs. HER2-positive: 21.4% vs. 33.6% vs. 45.9%, respectively, p < 0.001 χ2 test), as shown in Fig. 2. In addition, when focusing on HER2-negative cases, the significance of this relationship was maintained (HER2-0 vs. HER2-low-positive, p = 0.035 χ2 test). However, when considering each phenotypic subset (HR-positive/HER2-negative and TN), the association between lower pCR rates and HER2-low-positive phenotype was no longer significant (pCR rates in HR-positive/HER2-0 vs. HR-positive/HER2-low-positive: 12.1% vs. 8.3%, p = 0.721 χ2 test; TN/HER2-0 vs. TN/HER2-low-positive: 42.2% vs. 34.2%, p = 0.327 χ2 test).
In order to evaluate the proportion of HR-positive/HER2-low-positive breast cancer patients with high-risk features besides the failure to achieve pCR, the validated RPCB and CPS-EG scores were computed. Among patients with HR-positive/HER2-negative breast cancer and residual disease, RPCB and CPS-EG scores were available for 70 and 84 cases, respectively. The distribution of RPCB and CPS-EG classes according to HER2 expression on residual disease in shown in Table 4. In particular, among HR-positive/HER2-low-positive patients failing to achieve pCR with data available, 32.0% and 57.1% of had an estimated high risk of relapse based on RPCB class 3 and CPS-EG score ≥3, respectively.
HER2 evolution from baseline biopsy to residual disease after neoadjuvant treatment
The evolution of HER2 expression from baseline biopsy to residual disease after neoadjuvant chemotherapy in patients failing to achieve pCR is shown in Fig. 3. The overall rate of HER2 discordance was 26.4%, mostly represented by cases switching from HER2-0 to HER2-low-positive (n = 26, 8.9%) and from HER2-low-positive to HER2-0 phenotype (n = 43, 14.7%). In detail, among patients with HER2-0 phenotype on baseline biopsy, 33.8% (n = 26) experienced a conversion to HER2-low-positive phenotype, while 37.7% (n = 43) of HER2-low-positive breast cancer patients showed a conversion in the opposite direction. HER2-positive status was the most stable, with 2.7% (n = 8) of patients exhibiting either a gain (n = 1) or loss (n = 7) of HER2 positivity.
The evolution of HER2 expression in the HER2-negative cohort according to breast cancer phenotype is shown in Fig. 4. In particular, among HR-positive/HER2-negative breast cancer patients failing to achieve pCR (n = 95), the overall rate of HER2 discordance was 39%. In detail, 16.8% (n = 16) patients showed a conversion from HER2-0 primary breast cancer to HER2-low-positive residual disease, 21.1% showed a switch in the opposite direction (n = 20) and 1.1% (n = 1) exhibited the acquisition of HER2-positive status. Among triple-negative breast cancer patients failing to achieve pCR (n = 96), 10.4% (n = 10) showed a conversion from HER2-0 primary breast cancer to HER2-low-positive residual disease and 24.0% (n = 23) showed a switch in the opposite direction. None of the triple-negative breast cancer patients exhibited HER2-positivity gain.
These Sankey diagramas show the evolution of HER2 expression from baseline biopsy to residual disease after neoadjuvant chemotherapy in patients failing to achieve pCR in the HER2-negative cohort according to breast cancer phenotype. a Evolution of HER2 expression in HR+/HER2+subtype; b Evolution of HER2 expression in TN subtype. HR+ hormone-receptor positive, TN triple-negative.
Additionally, we evaluated the switch of hormone receptors from baseline biopsy to matched RD samples. The total rate of hormone-receptor discordance was 8.9% (n = 26). In particular, ER discordance rate was 6.2% (n = 18), with 2.0% of ER loss (n = 6) and 4.2% (n = 12) of ER gain; PgR discordance rate was 20.2% (n = 58), encompassing 12.9% rate of PgR loss (n = 37) and 7.3% of PgR gain (n = 21).
Exploratory survival analysis
Exploratory survival analysis did not reveal any statistically significant disease-free-survival (DFS) difference according to baseline HER2 expression. In particular, similar DFS rates were observed between HER2-0 and HER2-low-positive BC cohorts (HR 0.79, 95% CI 0.51–1.21, p = 0.27 log-rank test and Cox-regression model; Supplementary Fig. 1A). Similarly, no differences in DFS were observed according to HER2 expression when separately evaluating HR-positive/HER2-negative subgroup (HR 0.74, 95% CI 0.39–1.41, p = 0.35 log-rank test and Cox-regression model, Supplementary Fig. 1B) and triple-negative subgroup (HR 0.92, 95% CI 0.52–1.65, p = 0.79 log-rank test and Cox-regression model; Supplementary Fig. 1C).
In addition, when evaluating the potential prognostic impact of HER2 expression change from baseline biopsy to residual disease, focusing on HER2-negative cases, there was no DFS difference for concordance vs. discordance (HR 1.26, 95% CI 0.81–1.95, p = 0.29 log-rank test and Cox-regression model), concordant HER2–0 vs. gain of HER2-low-positive expression (HR 1.10, 95% CI 0.57–2.12, p = 0.77 log-rank test and Cox-regression model) or concordant HER2-low-positive vs. loss of HER2-low-positive expression (HR 1.43, 95% CI 0.79–2.57, p = 0.23 log-rank test and Cox-regression model), as shown in Supplementary Fig. 2A–C, respectively.
Discussion
In our large cohort of 446 breast cancer patients undergoing neoadjuvant treatment, we confirmed the strong relationship between HER2-low-positive breast cancer and HR-positive status, thus strengthening the possible crucial role or ER signaling in shaping HER2-low-positive breast cancer biology5,17,18,19,20,21. These findings are mirrored in recently reported gene expression analyses, that revealed HR-positive/HER2-negative cases with HER2-low-positive expression being more likely profiled as Luminal by PAM50-based intrinsic subtyping5,6,22.
In addition, we observed significantly lower pCR rates in patients with HER2-low-positive phenotype as compared to HER-0. This observation appears consistent with what has been recently reported in a pooled-analysis of individual data from four prospective trials, where HER2-low-positive patients experienced significantly lower pCR than those with HER2-0 breast cancer in the overall cohort and in HR-positive subgroup6. However, in our study, when assessing pCR rates separately in HR-positive/HER2-negative and TN subgroups, the association between HER2 expression and pCR was no longer significant, thus suggesting that, in our HER2-negative cohort, the major determinant of chemo-sensitivity was HR status rather than HER2 expression. Indeed, as expected, we observed HR-positive/HER2-negative patients having significantly lower pCR than the TN subgroup and, given the significant enrichment of the HER2-low-positive subgroup for HR-positive cases, these latter could have driven the lower rate of pCR of the overall HER2-low-positive cohort.
The main objective of this work was to explore the evolution of HER2-low-positive expression from baseline tumor to residual disease in patients undergoing neoadjuvant chemotherapy, by adopting a HER2-based three-tier algorithm. We observed a 26.4% overall rate of HER2 discordance from baseline biopsies to residual disease samples and this phenomenon mostly reflected the conversion to or from HER2-low-positive expression. Of note, we previously reported HER2 expression being highly unstable during disease evolution from primary breast cancer to relapse, mainly due to HER2-low-positive cases switching either from or to HER2-0 expression and findings from the present work solidify the great instability of HER2-low-positive expression in a different setting. Interestingly, all HER2-positive breast cancer patients exhibiting HER2-loss after neoadjuvant treatment converted to HER2-low-positive phenotype on residual disease, while none of them experienced a complete loss of HER2 expression.
There are several implications of our results.
Firstly, this susceptibility of HER2-low-positive expression to conversion after the exposure to neoadjuvant treatment adds to available evidence suggesting HR and/or HER2 status discordance from primary tumor to residual disease after neoadjuvant treatment as a relatively frequent phenomenon12,13,14,15,16. In this context, if, from one hand, our findings emphasize the importance to re-profile the tumor on residual disease, on the other, they support the inclusion of the HER2-low-positive category in this evaluation. It should however be noted that in our cohort, all patients underwent chemotherapy, thus precluding the possibility to uncovering whether the instability of HER2-low-positive expression reflects a genuine shift as a consequence of chemotherapy exposure or rather an analytical distortion. In this context, although the diagnostic accuracy of HER2 evaluation on core-needle biopsies has been suggested to be high as compared to surgical specimen23,24,25, this conclusion has been drawn by focusing on the dichotomization between HER2-positive vs. HER2-negative status. We have previously reported higher proportion of HER2-low-positive cases when primary tumor phenotype was assessed on core-needle biopsies as compared to treatment-naive surgical specimens, with pre-analytical variables and intratumor heterogeneity of HER2 expression both representing possible contributing factors to such analytical variability. Of course, this area might warrant further investigation8.
Secondly, if ongoing late-phase trials will confirm the positive results from early-phase clinical trials of novel anti-HER2 strategies for HER2-low-positive advanced breast cancer patients (NCT03734029 —Destiny-Breast04 met its primary endpoint—data not presented yet, NCT04494425—Destiny-Breast06), it is expected a rapid transfer of this experimental scenario in the early setting. In this context, our findings anticipate the forthcoming and, at that point, imperative need to broaden the pool of patients who may get access to anti-HER2 blockade in the context of clinical trials as well as proper selecting those who may potentially derive the greatest benefit from these novel strategies26. Indeed, we identified more than one third of patients with HER2-0 phenotype at baseline showing a conversion to HER2-low-positive expression after neoadjuvant treatment, thus suggesting that the evaluation of HER2 expression on residual disease may allow the access to potentially effective novel treatment strategies in a not negligible proportion of patients who would otherwise be excluded based on the primary tumor phenotype. In addition to that, when focusing on HR-positive/HER2- breast cancer patients showing HER2-low-positive expression on residual disease, we observed a high proportion of patients being classified as having high-risk features based on the previously validated prognostic scores RPCB and CPS-EG score. Indeed, while the mere presence of residual disease after neoadjuvant chemotherapy has been endorsed as a fit criterion for selecting high-risk triple-negative breast cancer patients for clinical trials testing escalated post-neoadjuvant strategies, this might not be the more reliable strategy for selecting high-risk patients with HR-positive/HER2- breast cancer, given the known sub-optimality of pCR as a surrogate prognostic biomarker in this breast cancer subtype27. Indeed, in recent years, several biomarkers considering not only the burden of residual disease but also its biology have been suggested as superior to pCR in terms of prognostic stratification. In this context, we decided to adopt CPS-EG score and RPCB for this purpose since they both have been validated specifically in the HR-positive/HER2- breast cancer subtype17,18,19,20, allowing us to identify HR-positive/HER2-low-positive breast cancer patients who may be defined at high risk and hence be potentially selected for post-neoadjuvant trials testing novel anti-HER2 ADCs as escalated strategies.
Another point deserving further discussion is that 7% of patients with HER2-positive breast cancer at baseline with no pCR, lost HER2 positivity, exhibiting HER2-low-positive phenotype on residual disease. Current evidence suggests that HER2-loss after neoadjuvant treatment may confer an additional negative prognostic trait in HER2-positive breast cancer patients already defined at high-risk of relapse based on the failure to achieve pCR12,16. Indeed, although subgroup analyses from the KATHERINE trial, which established TDM1 as the new standard of care in the post-neoadjuvant setting in patients failing to achieve pCR28, revealed that patients with HER2-loss at surgery still seemed to benefit from TDM1 over trastuzumab29, it is currently largely unknown whether those maintaining some level of HER2 expression (HER2-low-positive subgroup) may derive greater advantage by the administration of novel anti-HER2 ADCs given post-neoadjuvantly with respect to TDM1. Of course, translational analyses from an ongoing clinical trial comparing post-neoadjuvant TDM1 vs. Trastuzumab-Deruxtecan (NCT04622319) may be able to shed light in this unexplored area.
Exploratorily, we also performed an evaluation of hormone-receptor changes from baseline biopsy to residual disease after neoadjuvant therapy, observing an overall rate of discordance of 8.9%, thus further consolidating the value of biomarker status re-evaluation after neoadjuvant treatment in case of no-pCR. Within this framework, consistently with available evidence, we confirmed PgR being more prone to discordance than ER24,30.
Finally, an exploratory survival analysis was also conducted, which did not reveal any significant DFS difference between HER2-0 vs. HER2-low-positive expression at baseline, thus fueling the already existing uncertainty regarding the actual clinical distinctiveness of HER2-low-positive expression from a prognostic point of view5,6,7,26,31. Interestingly, HER2 expression discordance (nor either HER2-low-positive expression loss or gain) from baseline biopsy to residual disease after neoadjuvant chemotherapy did not retain any prognostic role in terms of DFS in HER2-negative breast cancer patients. These data stress the notion that the most relevant implication of retesting HER2 expression on residual disease by also including the HER2-low-positive category, might be that of enhancing the access to potentially effective drugs in patients at high-risk of relapse.
The present works presents several strengths. Firstly, this represents a study evaluating, in a large cohort of patients undergoing neoadjuvant chemotherapy, HER2 expression evolution from primary breast cancer to residual disease by incorporating the up-and-coming category of HER2-low-positive breast cancer. Secondly, although HER2 expression data reflected the local evaluation according to ASCO/CAP recommendations endorsed at the time of diagnosed, all cases diagnosed between 2007 and 2013 were reviewed to comply with the currently accepted 10% cutoff of cell staining for HER2 positivity, given that in 2007 this cutoff was raised to 30% before being restored to the original 10% in 2013. Thirdly, we decided to embrace the terminology recently suggested as a basis for a future international consensus for breast cancer classification according to HER2 expression6. Another possible strength of the present study is represented by the adoption of a 10% cutoff for distinguishing HR-positive vs. HR- cases. Indeed, although a major debate is still ongoing with regard to the optimal ER expression threshold for defining the access to endocrine therapy32 a mounting body of evidence suggests that patients harboring ER levels ranging from 1 to 10% (the so-called ER-low breast cancer) are phenotypically more similar to TN than HR-positive breast cancer33,34,35,36.
A final consideration regards the analytical reliability of IHC/ISH methods for detecting low levels of HER2 expression. In fact, on one hand, with the emergence of novel treatment strategies directed to patients with HER2-low-positive breast cancer, a stricter adherence to FDA/ASCO-CAP rules for HER2 scoring would be advisable, especially in the light of the suboptimal inter-pathologist agreement rates recently reported with regards to the distinction between IHC scores 0 and 1+5. On the other hand, the actual sensitivity of a semiquantitative assay, as IHC, in detecting low levels of HER2 expression may be questioned, and it is not inconceivable to hypothesize that a proportion of score = 0 by IHC may reflect an artefactual limitation rather than a genuine total absence of HER2 expression. On this ground, one might question to which extent low levels of HER2 expression should be considered too low to grant access to novel anti-HER2 agents. In this context, it might be worth investigating alternative techniques for the quantitative evaluation of HER2 expression, including those based on gene expression and proteomics analyses, some of which have already proven to be promising at this purpose37,38,39,40,41,42.
The major limitation of the present work is its retrospective nature, which may have been responsible for both selection and information bias. Another limitation is represented by the heterogeneity of neoadjuvant treatments. However, the impact of this flaw is downsized by the fact that almost 80% of the patients received anthracycline-taxane-based neoadjuvant chemotherapy, which currently represents the standard chemotherapy backbone in this setting. Finally, a central HER2 expression revision of all cases was not planned, however a good agreement with the original report was obtained after 100 random samples were reviewed by an expert pathologist in a blinded fashion.
In conclusion, we reported a remarkable instability of HER2 expression from primary breast cancer to residual disease in a large cohort of patients undergoing neoadjuvant chemotherapy, with HER2-low-positive expression instability being the major driver of such phenomenon. Our results encourage the reprofiling of residual disease by including the HER2-low category.
Materials and methods
Population
Breast cancer patients undergoing neoadjuvant chemotherapy at The Oncology Department of the Istituto Oncologico Veneto—IRCCS, Padova Italy, between January 2002 and June 2018 were identified. Cases for which HER2 status was available on baseline biopsy and, in case of residual disease, on matched surgical samples were included. Patients’ clinicopathologic features including age, stage at diagnosis, baseline HR status/expression and HER2 status and expression, type of neoadjuvant treatment, pathologic response (pCR vs. no-pCR), HR status/expression and HER2 status on residual disease (in case of no-pCR) were recorded.
Pathology
Cases were considered as HER2-positive in case of IHC score 3+ and/or HER2 gene amplification by ISH, HER2-low-positive in case of IHC scores 1+ or 2+ in the absence of gene amplification by ISH and HER2-0 in case of IHC score zero. In the present study we complied with the terminology proposed by Denkert et al. in their pivotal study on HER2-low-positive landscape in early breast cancer6. HER2 expression was retrospectively retrieved from pathology records and evaluated according to ASCO/CAP recommendations in place at the time of diagnosis (the IHC assays for HER2 adopted were all FDA approved and were: until 2008 Hercept-Test®, DAKO [ref. number 2007333; 1:1000 dilution]; 2009–2011 Pathway®, Ventana [clone 4B5] [ref. number 780–4422; 1:3000 dilution]; since 2012 Bond Oracle®, Leica [clone CB11] [ref. number 780–4422, 1:1000 dilution])). However, given the temporary endorsement of the 30% cutoff of cell staining for defining HER2 positivity by ASCO/CAP recommendation in place from 2007 to 2013, all cases diagnosed in this time interval were reviewed by one expert pathologist to comply with the currently adopted 10% cutoff. In addition, 100 random matched cases underwent blinded revision by one expert pathologist, achieving an 85% agreement with the original report.
Estrogen receptor (ER) and progesterone receptor (PgR) expression were retrieved from the original pathology records. A 10% cutoff of ER/PgR expression was adopted for defining ER/PgR-positive cases. Cases were considered HR-positive in case of ER and/or PgR positivity, and negative in case of both ER and PgR negativity.
Residual proliferative cancer burden (RPCB) was evaluated in HR-positive/HER2-negative cohort by combining residual cancer burden (RCB) and post-treatment Ki67, as previously described41,43 and validated44. CPS-EG score was calculated based on clinical stage, pathological stage, grade, and ER status45,46.
Statistical analysis
IBM SPSS Statistics (version 22.0) software (IBM Corp, Armonk, NY, USA) was used to carry out statistical analyses.
Patient demographics and clinical characteristics were reported by applying descriptive statistics. In particular, for continuous variables mean, median, range values, and quartiles were calculated. Distribution of continuous variables across subgroups was assessed by applying Student-t test, and The Mann–Whitney and Kolmogorov–Smirnov nonparametric tests. Chi-squared test (χ2) was applied to compare categorical variables across groups.
The evolution of HER2 expression from primary breast cancer to residual disease after neoadjuvant treatment was graphically reported by building Sankey diagrams. pCR was defined as the absence of invasive residual disease in both breast and lymph-nodes (ypT0/is, ypN0). DFS was defined as the time from diagnosis to recurrence or death from any cause. Survival curves were estimated by applying the Kaplan–Meier method and the log-rank test was used to test for differences between groups. The Cox-regression model was applied to calculate hazard ratios and 95% confidence intervals. All reported p-values are two-sided, and significance level was set at p < 0.05.
Ethical statement
In the present study tumor samples were collected after approval by the Institutional Review Board (Istituto Oncologico Veneto I.O.V.–IRCCS, Padova) and in accordance with the Declaration of Helsinki. All patients provided written-informed consent prior to inclusion into the study.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The datasets that support the findings of this study are not publicly available in order to protect patient privacy. The data will be available on reasonable request from the corresponding author: V.G., valentina.guarneri@unipd.it.
References
Banerji, U. et al. Trastuzumab duocarmazine in locally advanced and metastatic solid tumours and HER2-expressing breast cancer: a phase 1 dose-escalation and dose-expansion study. Lancet Oncol. 20, 1124–1135 (2019).
Modi, S. et al. Antitumor activity and safety of trastuzumab deruxtecan in patients with HER2-low-expressing advanced breast cancer: results from a phase Ib Study. J. Clin. Oncol. 38, 1887–1896 (2020).
Pistilli, B. et al. Clinical activity of MCLA-128 (zenocutuzumab) in combination with endocrine therapy (ET) in ER+/HER2-low, non-amplified metastatic breast cancer (MBC) patients (pts) with ET-resistant disease who had progressed on a CDK4/6 inhibitor (CDK4/6i). J. Clin. Oncol. 38(May), 1037–1037 (2020).
Eiger, D., Agostinetto, E., Saude-Conde, R. & de Azambuja, E. The exciting new field of HER2-low breast cancer treatment. cancers (Basel) 13, https://doi.org/10.3390/cancers13051015 (2021).
Schettini, F. et al. Clinical, pathological, and PAM50 gene expression features of HER2-low breast cancer. NPJ Breast Cancer 7, 1-020–00208-2 (2021).
Denkert, C. et al. Clinical and molecular characteristics of HER2-low-positive breast cancer: pooled analysis of individual patient data from four prospective, neoadjuvant clinical trials. Lancet Oncol. 22, 1151–1161 (2021).
Tarantino, P. et al. HER2-low breast cancer: pathological and clinical landscape. J. Clin. Oncol. 38, 1951–1962 (2020).
Miglietta, F. et al. Evolution of HER2-low expression from primary to recurrent breast cancer. NPJ Breast Cancer 7, 137-021–00343-4 (2021).
Miglietta, F., Dieci, M. V., Griguolo, G. & Guarneri, V. Neoadjuvant approach as a platform for treatment personalization: focus on HER2-positive and triple-negative breast cancer. Cancer Treat. Rev. 98, 102222 (2021).
Guarneri, V., Frassoldati, A., Giovannelli, S., Borghi, F. & Conte, P. Primary systemic therapy for operable breast cancer: a review of clinical trials and perspectives. Cancer Lett. 248, 175–185 (2007).
Prowell, T. M., Beaver, J. A. & Pazdur, R. Residual disease after neoadjuvant therapy—developing drugs for high-risk early breast cancer. N. Engl. J. Med. 380, 612–615 (2019).
Mittendorf, E. A. et al. Loss of HER2 amplification following trastuzumab-based neoadjuvant systemic therapy and survival outcomes. Clin. Cancer Res. 15, 7381–7388 (2009).
Tural, D. et al. Receptor discordances after neoadjuvant chemotherapy and their effects on survival. J. BUON. 24, 20–25 (2019).
van de Ven, S., Smit, V. T., Dekker, T. J., Nortier, J. W. & Kroep, J. R. Discordances in ER, PR and HER2 receptors after neoadjuvant chemotherapy in breast cancer. Cancer Treat. Rev. 37, 422–430 (2011).
Niikura, N. et al. Changes in tumor expression of HER2 and hormone receptors status after neoadjuvant chemotherapy in 21,755 patients from the Japanese breast cancer registry. Ann. Oncol. 27, 480–487 (2016).
Guarneri, V. et al. Loss of HER2 positivity and prognosis after neoadjuvant therapy in HER2-positive breast cancer patients. Ann. Oncol. 24, 2990–2994 (2013).
Sapino, A. et al. Gene status in HER2 equivocal breast carcinomas: impact of distinct recommendations and contribution of a polymerase chain reaction-based method. Oncologist 19, 1118–1126 (2014).
Ballard, M. et al. ‘Non-classical’ HER2 FISH results in breast cancer: a multi-institutional study. Mod. Pathol. 30, 227–235 (2017).
Rossi, V. et al. Moderate immunohistochemical expression of HER-2 (2+) without HER-2 gene amplification is a negative prognostic factor in early breast cancer. Oncologist 17, 1418–1425 (2012).
Osborne, C. K., Shou, J., Massarweh, S. & Schiff, R. Crosstalk between estrogen receptor and growth factor receptor pathways as a cause for endocrine therapy resistance in breast cancer. Clin. Cancer Res. 11, 865s–870ss (2005).
Ithimakin, S. et al. HER2 drives luminal breast cancer stem cells in the absence of HER2 amplification: implications for efficacy of adjuvant trastuzumab. Cancer Res. 73, 1635–1646 (2013).
Agostinetto, E. et al. HER2-low breast cancer: molecular characteristics and prognosis. Cancers (Basel) 13, https://doi.org/10.3390/cancers13112824 (2021).
You, K. et al. Comparison of core needle biopsy and surgical specimens in determining intrinsic biological subtypes of breast cancer with immunohistochemistry. J. Breast Cancer 20, 297–303 (2017).
Arnedos, M. et al. Discordance between core needle biopsy (CNB) and excisional biopsy (EB) for estrogen receptor (ER), progesterone receptor (PgR) and HER2 status in early breast cancer (EBC). Ann. Oncol. 20, 1948–1952 (2009).
Wolff, A. C. et al. HER2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update Summary. J. Oncol. Pract. 14, 437–441 (2018).
Dieci, M. V. & Miglietta, F. HER2: a never ending story. Lancet Oncol. 22, 1051–1052 (2021).
Cortazar, P. et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 384, 164–172 (2014).
von Minckwitz, G. et al. Trastuzumab emtansine for residual invasive HER2-positive. Breast Cancer N. Engl. J. Med. 380, 617–628 (2019).
Denkert, C. et al. Biomarker data from KATHERINE: a phase III study of adjuvant trastuzumab emtansine (T-DM1) versus trastuzumab (H) in patients with residual invasive disease after neoadjuvant therapy for HER2-positive breast cancer. J. Clin. Oncol. 38(May), 502–502 (2020). (ASCO Virtual Meeting 2020).
Zattarin, E. et al. Hormone receptor loss in breast cancer: molecular mechanisms, clinical settings, and therapeutic implications. Cells 9, 2644 (2020).
Domergue, C. et al. Impact of HER2 status (HER2-low versus HER2-0) on complete histologic response after neoadjuvant chemotherapy in early triple-negative breast cancer (TNBC). ESMO Congress 2021, abstract 156P (2021).
Burstein, H. J. et al. Customizing local and systemic therapies for women with early breast cancer: the St. Gallen International Consensus Guidelines for treatment of early breast cancer 2021. Ann. Oncol. 32, 1216–1235 (2021).
Iwamoto, T. et al. Estrogen receptor (ER) mRNA and ER-related gene expression in breast cancers that are 1% to 10% ER-positive by immunohistochemistry. J. Clin. Oncol. 30, 729–734 (2012).
Villegas, S. L. et al. Therapy response and prognosis of patients with early breast cancer with low positivity for hormone receptors-an analysis of 2765 patients from neoadjuvant clinical trials. Eur. J. Cancer 148, 159–170 (2021).
Viale, G. et al. Prognostic and predictive value of centrally reviewed expression of estrogen and progesterone receptors in a randomized trial comparing letrozole and tamoxifen adjuvant therapy for postmenopausal early breast cancer: BIG 1-98. J. Clin. Oncol. 25, 3846–3852 (2007).
Dieci, M. V. et al. Impact of estrogen receptor levels on outcome in non-metastatic triple negative breast cancer patients treated with neoadjuvant/adjuvant chemotherapy. NPJ Breast Cancer 7, 101-021–00308-7 (2021).
Ho-Pun-Cheung, A. et al. Quantification of HER1, HER2 and HER3 by time-resolved Forster resonance energy transfer in FFPE triple-negative breast cancer samples. Br. J. Cancer 122, 397–404 (2020).
Yardley, D. A. et al. Quantitative measurement of HER2 expression in breast cancers: comparison with ‘real-world’ routine HER2 testing in a multicenter Collaborative Biomarker Study and correlation with overall survival. Breast Cancer Res. 17, 41-015–0543-x (2015).
Jensen, K., Krusenstjerna-Hafstrom, R., Lohse, J., Petersen, K. H. & Derand, H. A novel quantitative immunohistochemistry method for precise protein measurements directly in formalin-fixed, paraffin-embedded specimens: analytical performance measuring HER2. Mod. Pathol. 30, 180–193 (2017).
Gustavson, M. D. et al. Standardization of HER2 immunohistochemistry in breast cancer by automated quantitative analysis. Arch. Pathol. Lab. Med. 133, 1413–1419 (2009).
Larson, J. S. et al. Analytical validation of a highly quantitative, sensitive, accurate, and reproducible assay (HERmark) for the measurement of HER2 total protein and HER2 homodimers in FFPE breast cancer tumor specimens. Pathol. Res. Int. 2010, 814176 (2010).
Chen, H. et al. Proteomic characterization of Her2/neu-overexpressing breast cancer cells. Proteomics 10, 3800–3810 (2010).
Sheri, A. et al. Residual proliferative cancer burden to predict long-term outcome following neoadjuvant chemotherapy. Ann. Oncol. 26, 75–80 (2015).
Miglietta, F. et al. Validation of residual proliferative cancer burden as a predictor of long-term outcome following neoadjuvant chemotherapy in patients with hormone receptor-positive/human epidermal growth receptor 2-negative breast cancer. Oncologist 25, e1355–e1362 (2020).
Marme, F. et al. Utility of the CPS+EG staging system in hormone receptor-positive, human epidermal growth factor receptor 2-negative breast cancer treated with neoadjuvant chemotherapy. Eur. J. Cancer 53, 65–74 (2016).
Jeruss, J. S. et al. Combined use of clinical and pathologic staging variables to define outcomes for breast cancer patients treated with neoadjuvant therapy. J. Clin. Oncol. 26, 246–252 (2008).
Acknowledgements
The authors acknowledge the following funding: DOR funding from the University of Padova—Department of Surgery, Oncology and Gastroenterology BIRD 2019 (to V.G., M.V.D., P.F.C.), BIRD 2020 (to V.G., M.V.D., G.G.), DOR-1830512/18 (to M.V.D.), DOR-2194859/21 (to M.V.D.); Fondazione AIRC under 5 per mille 2019 (ID. 22759 program—G.L. P.F.C. and M.F.); Health Ministry and Veneto Region research program (NET-2016–02363853).
Author information
Authors and Affiliations
Contributions
All authors made substantial contributions to this paper: (1) Substantial contributions to the conception or design of the work or the acquisition, analysis or interpretation of the data (2) Drafting the work or revising it critically for important intellectual content (3) Final approval of the completed version (4) Accountability for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Each author approved the submitted version and agreed to be personally accountable for the author’s own contributions.
Corresponding author
Ethics declarations
Competing interests
F.M. reports personal fee from Roche outside the submitted work. M.F. received honoraria for consulting, advisory role, speaker bureau, and/or research funding from Astellas Pharma, QED Therapeutics, Diaceutics, Tesaro, Roche, Eli Lilly, and Novartis, all outside the submitted work. P.F.C. reports personal fees from Novartis, EliLilly, AstraZeneca, Tesaro, BMS, Roche, all outside the submitted work. V.G.: reports personal fees from Roche, Novartis, Eli Lilly, MSD outside the submitted work. M.V.D. reports personal fees from Lilly, Genomic Health, Novartis and Celgene, all outside the submitted work. All remaining authors have declared no conflicts of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Miglietta, F., Griguolo, G., Bottosso, M. et al. HER2-low-positive breast cancer: evolution from primary tumor to residual disease after neoadjuvant treatment. npj Breast Cancer 8, 66 (2022). https://doi.org/10.1038/s41523-022-00434-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41523-022-00434-w
- Springer Nature Limited
This article is cited by
-
Unveiling the mysteries of HER2-low expression in breast cancer: pathological response, prognosis, and expression level alterations
World Journal of Surgical Oncology (2024)
-
Discrimination between HER2-overexpressing, -low-expressing, and -zero-expressing statuses in breast cancer using multiparametric MRI-based radiomics
European Radiology (2024)
-
Pathological complete response, category change, and prognostic significance of HER2-low breast cancer receiving neoadjuvant treatment: a multicenter analysis of 2489 cases
British Journal of Cancer (2023)
-
HER2-Low Breast Cancer: a New Subtype?
Current Treatment Options in Oncology (2023)
-
The dynamics of HER2-low expression during breast cancer progression
Breast Cancer Research and Treatment (2023)