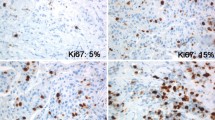
Abstract
Background
Although the prognostic value of Ki67 in breast cancer is well documented, using optimal cut-points for patient stratification, reproducibility of the scoring and interpretation of the results remains a matter of debate particularly when using tissue microarrays (TMAs). This study aims to assess Ki67 expression assessed on TMAs and their matched whole tissue sections (WTS). Moreover, whether the cut-off used for WTS is reproducible on TMA in BC molecular classes and the association between Ki67 expression cut-off, assessed on TMAs and WTS, and clinicopathological parameters and patient outcome were tested.
Method
A large series (n = 707) of primary invasive breast tumours were immunostained for Ki67 using both TMA and WTS and assessed as percentage staining and correlated with each other, clinicopathological parameters and patient outcome. In addition, MKI67 mRNA expression was correlated with Ki67 protein levels on WTS and TMAs in a subset of cases included in the METABRIC study.
Results
There was moderate concordance in Ki67 expression between WTS and TMA when analysed as a continuous variable (Intraclass correlation coefficient = 0.61) and low concordance when dichotomised (kappa value = 0.3). TMA showed low levels of Ki67 with mean percentage of expression of 35 and 22% on WTS and TMA, respectively. MKI67 mRNA expression was significantly correlated with protein expression determined on WTS (Spearman Correlation, r = 0.52) and to a lesser extent on TMA (r = 0.34) (p < 0.001). Regarding prediction of patient outcome, statistically significant differences were detected upon stratification of patients with tumours expressing Ki67 at 10, 15, 20, 25 or 30% in TMA. Using TMA, ≥20% Ki67 provided the best prognostic cut-off particularly in triple-negative and HER2-positive classes.
Conclusion
Ki67 expression in breast cancer can be evaluated using TMA although different cut-points are required to emulate results from WTS. A cut-off of ≥20% for Ki67 expression in BC provides the best prognostic correlations when TMAs are used.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ki67 has been extensively assessed and reported as a prognostic and predictive marker in invasive breast cancer (BC) [1,2,3,4,5,6,7]. High Ki67 expression in BC is associated with worse prognosis. In two meta-analyses published in 2007 and 2008, high Ki67 expression in both node-positive and node-negative invasive BC showed significantly worse overall and disease-free survival [8, 9]. Additionally, results of a systematic review support the role of Ki67 as a prognostic marker [10] and as an independent predictive factor for neoadjuvant chemotherapy in BC patients [5, 6, 11]. Furthermore, the St. Gallen consensus panel has recommended Ki67 as a marker for the definition of intrinsic BC subtypes to differentiate between luminal A and luminal B subgroups [12, 13].
In clinical practice, the evaluation of prognostic/predictive factors usually depends upon the stratification of the patients into distinct risk groups based on the status of such factor. The common approach is the choice of an optimal cut-off point for the prognostic/predictive factor, assessed as a continuous variable, e.g. percentage of cells stained, to define these groups. The optimal cut-point for Ki67 in BC is currently debatable despite the large number of published studies reporting significant results [14, 15]. The recent report on the second phase of the Ki67 trial reported that there was a need to standardise the pre-analytical and analytical features for Ki67 immunohistochemistry, so that it can be incorporated to drive patient-care decisions in clinical practice [16]. In 2009, the St. Gallen panel proposed that Ki67 expression should be stratified into three groups: low <15%, intermediate 16–30% and high >30% [17]. This was based on univariate analysis carried out with different Ki67 expression cut-points to find those best stratifying the patients with lowest significant p values according to survival using Ki67 immunoreactivity and standardised mitotic index [18]. In 2011, St. Gallen recommended an alternative KI67 cut-point at 14% in order to separate Oestrogen Receptor (ER)-positive tumours into luminal A (<14%) and luminal B (≥14%) [12]. This was derived from comparison with gene array data as a prognostic factor [19]. In 2013, St Gallen revised their threshold to ≥20% for ‘high’ Ki67 status with the option to also use locally specified cut-points [13]. Recently, at the 2015 St. Gallen Breast Cancer Conference, a median cut-off value of Ki67 within the range of 20–29% to differentiate ‘luminal B-like’ has been recommended [20]. As shown by Urruticoechea et al. [21], up to 17 studies that included more than 200 patients displayed statistically significant association between Ki67 and prognosis given that convincing evidence for a biological relationship. However, the cut-offs to discriminate a high from low level of Ki67 varied from 1 to 29%, consequently limiting its clinical utility. Furthermore, during the past decade, multiple research studies have additionally reported the assessment of Ki67 in BC using tissue microarrays (TMA) platform [14] [15], although it remains unclear as to their validity and comparison with assessment in whole tissue sections (WTS).
In this study, we aim to assess BC proliferative fraction using Ki67 assessment utilising matched cases prepared as TMA and WTS taking into account the optimal cut-off value for Ki67 assessed on TMA, the common method of proliferation assessment in the research setting on large cohorts. Herein we aimed at determining (1) to what extent Ki67 protein as well as transcriptome levels are matched between TMA and WTS; (2) whether the cut-point used for WTS is reproducible using TMA in different molecular classes. For the latter aim, the association between Ki67 expression cut-points assessed on TMAs and WTS and the standard clinicopathological variables and patient outcome was tested as endpoints.
Materials and methods
Patient cohort
This study was approved by the Nottingham Research Ethics Committee 2 under the title ‘Development of a molecular genetic classification of breast cancer’.
The expression of Ki67 was assessed on 707 cases of invasive BC cases using WTS and TMA. TMAs were prepared using 0.6-mm cores sampled from the invasive tumour edge as previously described [22]. Cases were derived from the retrospective Nottingham Tenovus Primary Breast Carcinoma Series. This is a consecutive well-defined series of early-stage primary operable invasive BC (TNM Stage I–III, excluding T3 and T4 tumours) from patients presented to Nottingham City Hospital from 1988 to 1998. The age of the patients was ≤70 years (Supplementary Table 1). Moreover, the clinical details of the patients including age and menopausal status as well as the tumour details including tumour size, grade, lymphovascular invasion (LVI) and lymph node status were also available and prospectively maintained. Survival data include Breast Cancer-Specific Survival (BCSS), in months, from the date the primary surgical treatment to the time of death from breast cancer. Molecular classes were defined as luminal (ER+ and/or PR+), HER2+ (HER2+ regardless of the expression of other markers) and triple-negative (TN; HER2−, ER− and PR−). In this cohort, transcriptomic data for MKI67 were available for a subset (n = 101) from Nottingham cases that were included in the METABRIC cohort [23].
Immunohistochemistry
4-μm sections were freshly cut from representative paraffin blocks and transferred onto slides (Surgipath Xtra Adhesive, Leica, Germany). Slides were incubated on a 60 °C hotplate for 10 min, followed by deparaffinisation and rehydration using xylene and graded alcohol. For antigen retrieval, sections were incubated in Citrate Buffer at pH 6.0 for 20 min using microwave. Manual immunohistochemistry staining was performed using either the Novolink™ Max Polymer Detection Kit (Leica, Newcastle, UK) for the TMAs and the standard streptavidin–biotin complex method for the WTS following manufacturer’s instructions and as previously described [4]. Optimised primary antibody, MIB-1 monoclonal mouse diluted 1:100 (Dako, Ref-M7240) antibody was applied and incubated for 1 h at room temperature. Finally, DAB chromogen reagent was incubated for 5 min, then 0.1% Haematoxylin was added as a counter stain. Dehydration, clearing, mounting and cover-slipping were performed as previously described. Human tonsil sections were used as a positive control, while negative controls were performed by omitting the application of primary antibody.
Ki67 assessment
Ki67-stained TMA slides were scanned into high-resolution digital images (0.45 µm/pixel) using a NanoZoomer slide scanner (Hamamatsu Photonics Welwyn Garden City, UK). Scoring of TMA was performed on digital images using a web-based interface (Distiller, Slidepath Ltd., Dublin, Ireland). Only the invasive breast cancer cells present in the TMA cores were assessed for Ki67 staining and scored as a percentage of the positively stained nuclei [15]. All tumour cell nuclei with homogenous granular staining, multiple speckled staining or nucleolar staining were regarded as positively stained regardless of their staining intensity [24]. To test for inter-observer concordance, three TMA slides (n = 350) were re-scored by another observer (MA). Scoring of WTS was performed in the areas with highest number of positive nuclei (hot spot) within the invasive component of the tumour as previously described [4]. Hot spots were identified by scanning the section for immunostaining evaluation using a light microscope at low power magnification (×100). Ki67 was expressed as the percentage of positive malignant cells in 1000 malignant cells assessed under high power magnification (×400). To assess for inter-observer concordance, a subset of cases (n = 180) was re-scored by another observer (AM).
Statistical analysis
Statistical analysis was performed using IBM SPSS software version 22 (SPSS Inc., Chicago, IL, USA). For all statistical tests a p value < 0.05 was considered significant. Spearman correlation test, Intraclass Correlation Coefficient (ICC) and kappa statistic were used to test the reproducibility and the correlation between the Ki67 assessment between TMA and WTS. In kappa, complete agreement is reflected by a value of 1.0 and only by chance alone results in a value of zero. Although in the literature there is no agreed standard criteria for kappa value that indicates adequate agreement, Landis and Koch proposed the following agreement measures for categorical data: kappa <0.00 represents poor agreement, 0.00–0.20 slight, 0.21–0.40 fair, 0.41–0.60 moderate, 0.61–0.80 substantial and 0.81–1.00 almost perfect agreement [25]. Accordingly, Mikami et al. suggested that based on the similarity to the kappa coefficient, ICC between 0.41 and 0.60 is considered as moderate correlation; 0.61–0.80 as substantial correlation and >0.80 as a perfect correlation [26]. Chi-square, Kaplan–Meier and Cox regression tests were applied to test the association with the standard clinicopathological parameters, other prognostic biomarkers and outcome of breast cancer patients. This study adheres to REporting recommendations for tumour MARKer prognostic studies (REMARK) criteria [27].
Results
Comparison of Ki67 expression between TMA and WTS (Protein expression)
Using WTS, Ki67 expression was not normally distributed (Supplementary Fig. 1A; range 0–99%): the mean percentage was 34.8%, while the median percentage was 20%. Similar to the distribution seen with WTS, Ki67 expression scored on TMAs was not normally distributed (Supplementary Fig. 1B; range 0–95%): the mean percentage was 21.9%, while the median was 10%. The reproducibility of Ki67 assessment on TMAs showed that there is a significant correlation (p < 0.001) between the two observers’ scoring. Agreement between the two observers showed an almost perfect concordance (p < 0.001) as tested by ICC intraclass correlation coefficient (ICC = 0.870, 95% confidence interval (CI) = 0.838–0.896). On the other hand, Ki67 scoring on WTS showed substantial concordance (ICC = 0.75, 95% CI = 0.670–0.815).
When Ki67 expression was compared between TMA and WTS, there was a significant correlation when measured as a continuous variable (p < 0.001), the Spearman’s correlations 0.50 with an r 2 value of 0.025 (Fig. 1). When ICC was used, substantial correlation was observed (ICC = 0.61, p < 0.001, 95% CI = 0.45–0.71).
To evaluate the reproducibility of Ki67 expression between WTS and TMA differing cut off points between platforms were assessed. The highest concordance was obtained when WTS is 10%. Therefore, for further analysis, the data were dichotomised at 10% as a fixed cut-point for WTS, which was published previously as the optimal cut-off [28] as well as, and at different cut-points for TMA to evaluate the reproducibility of the WTS Ki67 expression: 5, 15, 20, 25 and 30% (Table 1). As shown in Supplementary Fig. 2, higher cut-off values resulted in misclassification of a higher percentage (62.8%) of cases assessed on TMAs into the low proliferation group compared with their matched WTS. Conversely, lower cut-off values resulted in a higher number of cases matching between the positive cases. However, there was a high percentage (49.8%) of false-positive cases when assessed on TMA compared with WTS. A cut-off of 20% for Ki67 expression determined using TMAs seems to give the highest concordance in both positive and negative groups with less both false-positive and false-negative Ki67 expressions at 10% determined using WTS.
To test for the impact of cut-points on patient outcome as an end point, cases were classified based on their Ki67 expression, whether it was low or high, on TMAs and matched WTS. Therefore, using 10% as a cut-off for Ki67 on WTS and 20% as a cut-off for on TMAs, four groups were produced. Group one comprised cases with low Ki67 expression on TMAs and their matched WTS, group two comprised cases with high Ki67 expression on TMAs with low Ki67 expression on their matched WTS, group three cases with low Ki67 expression on TMAs with high Ki67 expression on their matched WTS and group four with high Ki67 expression on TMAs with high Ki67 expression on their matched WTS. Statistically significant differences were observed between these groups regarding patients outcome (Long Rank (LR) = 31.79, p < 0.001), (Fig. 2). Interestingly, there was no significant difference between the groups including high Ki67 expression on WTS (LR = 0.39, p = 0.52); therefore, they seem to have more or less similar poor outcome.
Comparison of Ki67 expression between TMA and WTS in different molecular subclasses
The studied cases were defined with regard to their molecular class as luminal, which includes ER positive cases; HER2+ and triple-negative (TN), which includes ER−, PR− and HER2− cases. Assessment of the concordance between Ki67 expression on TMAs and WTS in molecular subclasses using different cut-points showed that in TN and HER2+ tumours, a cut-off of 20% seemed to give the highest concordance between WTS and TMAs. In TN classes, the highest concordance between the positive cases (91.5%) with the lowest number of false positives (8.5%) was shown with 20% cut-point using TMA (Supplementary Fig. 3). Similarly, for HER2-positive tumours 20% was the optimal cut-off to classify tumour proliferation using TMAs where there was 96.2% concordance for the positive cases and 3.8% false-positive cases (Supplementary Fig. 4). However, in the luminal class, there was no optimal cut-off for Ki67 determined on TMAs which was reproducible to the Ki67 scoring on WTS (Supplementary Fig. 5).
Comparison of Ki67 expression on TMA and WTS with MKI67 mRNA expression
Using the METABRIC cohort, MKI67 mRNA data were available for 197 and 123 cases matched with WTS and TMA cases, respectively. The correlation between MKI67 mRNA and Ki67 protein expression determined using 197 cases of WTS was significant, p < 0.001 and Spearman’s correlation coefficient = 0.587. Although the correlation between MKI67 mRNA and 123 cases Ki67 assessed on TMAs was significant (p < 0.001), Spearman’s correlation was less compared with WTS = 0.343. Figure 3 shows the correlation between MKI67 mRNA with WTS (r 2 = 0.31) and TMA (r 2 = 0.17). In matching 101 BC cases assessed on both TMA and WTS, MKI67 mRNA expression was evaluated. Higher significance was observed for Ki67 assessed on WTS (Spearman’s correlation coefficient was 0.529 and p < 0.001) than on TMA (Spearman’s correlation coefficient was 0.341 and p < 0.001).
Ki67 and clinicopathological variables and patient outcome
When assessing Ki67 expression on TMA cores, high expression of Ki67 (>20%) was significantly associated with larger tumour size, higher grade, more nuclear pleomorphism, higher mitotic scores and less tubule formation (Supplementary Tables 2 and 3, p < 0.05). Regarding patient outcome, univariate survival analysis of Ki67 determined using TMAs showed that a cut-off of 20% was the most significantly associated with BCSS (LR = 8.76, p = 0.003, Fig. 4). Furthermore, using Cox regression analysis, different cut-offs points and the relations with BCSS were investigated (Table 2). Interestingly, 20% showed the highest risk on patients’ survival (hazards ratio, HR = 1.52, 95% CI = 1.15–2.0, p = 0.003). On the other hand, univariate survival analysis of Ki67 on WTS using Kaplan–Meier test showed that Ki67 at a cut-off of 10% was significantly associated with BCSS (LR = 30.1, p < 0.001). Furthermore, using Cox regression, >10% expression of Ki67 gave the highest risk on patients’ survival (HR = 2.95, 95% CI = 1.96–4.43, p < 0.001; Table 3).
Discussion
One of the attractive alternatives for using WTS section in the research field is the use of the TMAs since a large number of the tissue samples can be simultaneously analysed under the same experimental conditions. Additionally, it is a time, resource and cost effective [29, 30]. There is a mounting evidence indicating the usefulness TMAs in translational biomarker discovery/validation studies utilising materials from large scale population-based studies showing high concordance rates between TMA and WTS [31]. However, it is imperative to recognise its limitations especially in interpreting the results of biomarkers with considerable spatial intra-tumour heterogeneity of expression. In this study, our results assess different aspects regarding the comparison between the WTS and TMA demonstrating its relation to the reproducibility. There is a significant concordance between the Ki67 expression in the WTS and TMA. Importantly, concordance was substantial when continuous data are used (i.e. Ki67%) and much lower when dichotomised. The latter observation is probably due to the more tendency of TMA to give lower Ki67 estimates than the whole sections, which we observed in Ki67 scores of matched cases assessed on WTS and TMAs. In addition to the intratumoral heterogeneity, this is could be as a result of using one TMA core. Using more than one TMA core or a larger core diameter has been suggested to achieve better representation of the tumour proliferative fraction. Although Karlsson et al. [32] and Batistatou et al. [33] showed excellent agreement between TMAs and whole sections they have used only 10 and 88 cases of BC, respectively.
To assess the inter-observer reproducibility of Ki67 assessment, a significant correlation between the scorers was observed in Ki67 using TMAs. This result is consistent with results published by an international Ki67 reproducibility study which showed a high intra-laboratory reproducibility [15]. However, the same study resulted in only moderate reproducibility between different laboratories, which necessitates a standardised scoring methodology. The comparison between the two Ki67 assessed on WTS and TMA in relation to the clinicopathological parameters and BC-related biomarkers yielded comparably similar associations. As expected, high Ki67 was significantly associated with larger tumour size, higher tumour grade, more nuclear pleomorphism and mitotic scores.
Currently, consensus is lacking regarding an optimal cut-off for Ki67 expression both in the clinical setting and research settings. This affected the comparison of Ki67 expression in different clinical trials [15]. Ki67 has a significant prognostic value over a wide range of cut-offs and the optimisation of one cut-off is controversial. For instance, Urruticoechea et al. demonstrated, after evaluating of 18 studies, the wide range of Ki67 cut-points ranging from 1% to up to 29%. Accordingly, they concluded that this varied Ki67 cut-off may be the reason for its restricted clinical use [21]. One possible explanation for the wide range of cut-offs could be the absence of standardisation in the pre-analytical tissue handling, in terms of duration of ischaemia, time to fixation, dilution and pH of formalin used in tissue fixation and procedures of antigen retrieval which largely depend on the pre-analytical phase. Pathologist’s scoring of the immunohistochemical staining also has a minor role [34]. Therefore, standardised approaches in the pre-analytical tissue handling, especially adequate fixation, are crucial for reliable proliferative fraction assay.
In the current study, we evaluated a wide range of cut-points in the studied series, and all were significant with patient outcome as the study end point. The same cut-off points (10, 15, 20, 25 and 30%) have been examined in the comparison of Ki67 on WTS and all gave significant results with BCSS. Interestingly, there are different cut-offs that have the best correlation with clinicopathological parameters, biomarkers and patients outcome according to the type of tissue used, WTS or TMA. 10% seems to be the best cut-off when the WTS was used, while the statistical significance was higher using 20% as cut-off when Ki67 was assessed on TMAs. This cut-off of 10% was previously used in several series published by others for different purposes. For instance, Pathmanathan et al. evaluated the utility of Ki67 as a prognostic marker in a series of patients and emphasised that the highest sensitivity and specificity of Ki67 cut-off is 10% after evaluation of different cut-offs using 203 cases as WTS [35]. Furthermore, Shui et al. using BC cases processed as WTS concluded that assessment of Ki67 at 10% is a candidate for a standard method in breast cancer clinical practice [36]. Importantly and supporting to our results suing TMAs, the St Gallen has revised the threshold for ‘high’ Ki67 status to ≥20% with the option to also use locally specified cut-points [13].
Conclusions
Ki67 expression can be evaluated using WTS and TMA; however, due to the reported substantial heterogeneity of Ki67 expression in BC the latter should be interpreted with caution. Assessment of Ki67 as a continuous variable may better reflect the proliferative status than the predefined dichotomised values currently in use. A cut-point of 20% in BC when assessing Ki67 on TMAs appears to be optimum both at concordance with WTS as well as with patients’ outcome.
References
Trihia H, Murray S, Price K, Gelber RD, Golouh R, Goldhirsch A, Coates AS, Collins J, Castiglione-Gertsch M, Gusterson BA (2003) Ki-67 expression in breast carcinoma: its association with grading systems, clinical parameters, and other prognostic factors—a surrogate marker? Cancer 97(5):1321–1331
Domagala W, Markiewski M, Harezga B, Dukowicz A, Osborn M (1996) Prognostic significance of tumor cell proliferation rate as determined by the MIB-1 antibody in breast carcinoma: its relationship with vimentin and p53 protein. Clin Cancer Res 2(1):147–154
Viale G, Giobbie-Hurder A, Regan MM, Coates AS, Mastropasqua MG, Dell’Orto P, Maiorano E, MacGrogan G, Braye SG, Ohlschlegel C et al (2008) Prognostic and predictive value of centrally reviewed Ki-67 labeling index in postmenopausal women with endocrine-responsive breast cancer: results from Breast International Group Trial 1–98 comparing adjuvant tamoxifen with letrozole. J Clin Oncol 26(34):5569–5575
Aleskandarany MA, Green AR, Rakha EA, Mohammed RA, Elsheikh SE, Powe DG, Paish EC, Macmillan RD, Chan S, Ahmed SI et al (2010) Growth fraction as a predictor of response to chemotherapy in node-negative breast cancer. Int J Cancer 126(7):1761–1769
Brown JR, DiGiovanna MP, Killelea B, Lannin DR, Rimm DL (2014) Quantitative assessment Ki-67 score for prediction of response to neoadjuvant chemotherapy in breast cancer. Lab Invest 94(1):98–106
Ingolf JB, Russalina M, Simona M, Julia R, Gilda S, Bohle RM, Andrea H, Erich S, Daniel H (2014) Can ki-67 play a role in prediction of breast cancer patients’ response to neoadjuvant chemotherapy? Biomed Res Int 2014:628217
Takei H, Kurosumi M, Yoshida T, Hayashi Y, Higuchi T, Uchida S, Ninomiya J, Oba H, Inoue K, Nagai S et al (2011) Neoadjuvant endocrine therapy of breast cancer: which patients would benefit and what are the advantages? Breast Cancer (Tokyo, Japan) 18(2):85–91
de Azambuja E, Cardoso F, de Castro G Jr., Colozza M, Mano MS, Durbecq V, Sotiriou C, Larsimont D, Piccart-Gebhart MJ, Paesmans M (2007) Ki-67 as prognostic marker in early breast cancer: a meta-analysis of published studies involving 12,155 patients. Br J Cancer 96(10):1504–1513
Stuart-Harris R, Caldas C, Pinder SE, Pharoah P (2008) Proliferation markers and survival in early breast cancer: a systematic review and meta-analysis of 85 studies in 32,825 patients. Breast 17(4):323–334
Luporsi E, Andre F, Spyratos F, Martin PM, Jacquemier J, Penault-Llorca F, Tubiana-Mathieu N, Sigal-Zafrani B, Arnould L, Gompel A et al (2012) Ki-67: level of evidence and methodological considerations for its role in the clinical management of breast cancer: analytical and critical review. Breast Cancer Res Treat 132(3):895–915
Yerushalmi R, Woods R, Ravdin PM, Hayes MM, Gelmon KA (2010) Ki67 in breast cancer: prognostic and predictive potential. Lancet Oncol 11(2):174–183
Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thurlimann B, Senn HJ, Panel M (2011) Strategies for subtypes—dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol, England 22:1736–1747
Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thürlimann B, Senn HJ, Panel members (2013): Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol 24(9):2206–2223
Dowsett M, Nielsen TO, A’Hern R, Bartlett J, Coombes RC, Cuzick J, Ellis M, Henry NL, Hugh JC, Lively T et al (2011) Assessment of Ki67 in breast cancer: recommendations from the International Ki67 in Breast Cancer working group. J Natl Cancer Inst 103(22):1656–1664
Polley MY, Leung SC, McShane LM, Gao D, Hugh JC, Mastropasqua MG, Viale G, Zabaglo LA, Penault-Llorca F, Bartlett JM et al (2013) An international Ki67 reproducibility study. J Natl Cancer Inst 105(24):1897–1906
Denkert C, Budczies J, von Minckwitz G, Wienert S, Loibl S, Klauschen F (2015) Strategies for developing Ki67 as a useful biomarker in breast cancer. Breast 24(Suppl 2):S67–S72
Goldhirsch A, Ingle JN, Gelber RD, Coates AS, Thurlimann B, Senn HJ (2009) Thresholds for therapies: highlights of the St Gallen International Expert Consensus on the primary therapy of early breast cancer 2009. Ann Oncol 20(8):1319–1329
Jalava P, Kuopio T, Juntti-Patinen L, Kotkansalo T, Kronqvist P, Collan Y (2006) Ki67 immunohistochemistry: a valuable marker in prognostication but with a risk of misclassification: proliferation subgroups formed based on Ki67 immunoreactivity and standardized mitotic index. Histopathology 48(6):674–682
Cheang MC, Chia SK, Voduc D, Gao D, Leung S, Snider J, Watson M, Davies S, Bernard PS, Parker JS et al (2009) Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst 101(10):736–750
Coates AS, Winer EP, Goldhirsch A, Gelber RD, Gnant M, Piccart-Gebhart M, Thurlimann B, Senn HJ (2015) Tailoring therapies-improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann Oncol 26(8):1533–1546
Urruticoechea A, Smith IE, Dowsett M (2005) Proliferation marker Ki-67 in early breast cancer. J Clin Oncol 23(28):7212–7220
Green AR, Powe DG, Rakha EA, Soria D, Lemetre C, Nolan CC, Barros FF, Macmillan RD, Garibaldi JM, Ball GR et al (2013) Identification of key clinical phenotypes of breast cancer using a reduced panel of protein biomarkers. Br J Cancer 109(7):1886–1894
Curtis C, Shah SP, Chin SF, Turashvili G, Rueda OM, Dunning MJ, Speed D, Lynch AG, Samarajiwa S, Yuan Y et al (2012) The genomic and transcriptomic architecture of 2000 breast tumours reveals novel subgroups. Nature 486(7403):346–352
Ahlin C, Aaltonen K, Amini RM, Nevanlinna H, Fjallskog ML, Blomqvist C (2007) Ki67 and cyclin A as prognostic factors in early breast cancer. What are the optimal cut-off values? Histopathology 51(4):491–498
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33(1):159–174
Mikami Y, Ueno T, Yoshimura K, Tsuda H, Kurosumi M, Masuda S, Horii R, Toi M, Sasano H (2013) Interobserver concordance of Ki67 labeling index in breast cancer: Japan Breast Cancer Research Group Ki67 ring study. Cancer Sci 104:1539–1543
McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM (2005) REporting recommendations for tumor MARKer prognostic studies (REMARK). Nat Clin Pract Oncol 2(8):416–422
Aleskandarany MA, Rakha EA, Macmillan RD, Powe DG, Ellis IO, Green AR (2011) MIB1/Ki-67 labelling index can classify grade 2 breast cancer into two clinically distinct subgroups. Breast Cancer Res Treat 127(3):591–599
Tzankov A, Went P, Zimpfer A, Dirnhofer S (2005) Tissue microarray technology: principles, pitfalls and perspectives—lessons learned from hematological malignancies. Exp Gerontol 40(8–9):737–744
Shergill IS, Shergill NK, Arya M, Patel HR (2004) Tissue microarrays: a current medical research tool. Curr Med Res Opin 20(5):707–712
Zhang D, Salto-Tellez M, Putti TC, Do E, Koay ES (2003) Reliability of tissue microarrays in detecting protein expression and gene amplification in breast cancer. Mod Pathol 16(1):79–85
Karlsson C, Bodin L, Piehl-Aulin K, Karlsson MG (2009) Tissue microarray validation: a methodologic study with special reference to lung cancer. Cancer Epidemiol, Biomark Prev 18(7):2014–2021
Batistatou A, Televantou D, Bobos M, Eleftheraki AG, Kouvaras E, Chrisafi S, Koukoulis GK, Malamou-Mitsi V, Fountzilas G (2013) Evaluation of current prognostic and predictive markers in breast cancer: a validation study of tissue microarrays. Anticancer Res 33(5):2139–2145
Colozza M, Sidoni A, Piccart-Gebhart M (2010) Value of Ki67 in breast cancer: the debate is still open. Lancet Oncol 11(5):414–415
Pathmanathan N, Balleine RL, Jayasinghe UW, Bilinski KL, Provan PJ, Byth K, Bilous AM, Salisbury EL, Boyages J (2014) The prognostic value of Ki67 in systemically untreated patients with node-negative breast cancer. J Clin Pathol 67(3):222–228
Shui R, Yu B, Bi R, Yang F, Yang W (2015) An interobserver reproducibility analysis of Ki67 visual assessment in breast cancer. PLoS ONE 10(5):e0125131
Acknowledgements
A Muftah is funded by Ministry of Higher Education and Scientific Research, Libya. M Aleskandarany and M Diez-Rodriguez are funded by Ha’il University, KSA. The authors thank the Nottingham Health Science Biobank, Nottingham City Hospital NHS trust, and the Breast Cancer Now Tissue Bank for the provision of tissues used in this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest.
Additional information
Mohammed A. Aleskandarany is the First Joint Author.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10549_2017_4270_MOESM2_ESM.jpg
Supplementary Fig. 2: Shows the percentage of cases classified as low/high proliferative assessed on TMAs and WTS using the whole cohort (n = 707). (JPEG 788 kb)
10549_2017_4270_MOESM3_ESM.tif
Supplementary Fig. 3: Shows the percentage of matched cases between TMA and WTS in the triple negative BC subtype. (TIFF 93 kb)
10549_2017_4270_MOESM4_ESM.tif
Supplementary Fig. 4: Shows the percentage of matched cases between TMA and WTS in the HER2 positive BC subtype. (TIFF 90 kb)
10549_2017_4270_MOESM5_ESM.tif
Supplementary Fig. 5: Shows the percentage of matched cases between TMA and WTS in the luminal BC subtype. (TIFF 120 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Muftah, A.A., Aleskandarany, M.A., Al-kaabi, M.M. et al. Ki67 expression in invasive breast cancer: the use of tissue microarrays compared with whole tissue sections. Breast Cancer Res Treat 164, 341–348 (2017). https://doi.org/10.1007/s10549-017-4270-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-017-4270-0