Abstract
Background
The incidence of brain metastases in breast cancer patients has increased in the last years. However, the knowledge about tumor cell invasion in the brain is still very limited. Based on our recent study on cDNA microarray data of breast cancer patients, we hypothesized that two enzymes involved in the hyaluronan metabolism, namely, hyaluronan synthase 2 (HAS2) and hyaluronidase 1 (HYAL1) are associated with brain metastases formation.
Methods
Protein expression levels of hyaluronan, HAS2, and HYAL1 were analyzed in primary breast cancer, and metastatic tissue samples from different localizations (brain, bone, skin, liver, and lung) were included in four different cohorts by immunohistochemistry. Correlations of expression levels with clinical and pathological parameters were performed within the individual cohorts.
Results
Higher HYAL1 expression was detected among primary tumors from patients with subsequent brain metastases compared with those without brain metastases (p = 0.011). Interestingly, brain metastatic tissue showed a significantly reduced HYAL1 expression compared with the corresponding primary tumor (p = 0.003). HYAL1 expression in brain metastases was also significantly lower than in skin, liver, and lung metastases. Further, hyaluronan staining in brain metastases was mainly located on the surface of the tumor cells, whereas in all other metastatic sites hyaluronan was only detected in the extracellular matrix. We could not show an association of HAS2 with the formation of brain metastases.
Conclusions
In conclusion, our results suggest that the enzyme HYAL1 plays a role in tumor dissemination and brain-specific colonization, rather than in subsequent metastatic out-growth.





Similar content being viewed by others
Abbreviations
- BM:
-
Brain metastases
- BC:
-
Breast cancer
- BBB:
-
Blood–brain-barrier
- ECM:
-
Extracellular matrix
- HA:
-
Hyaluronan
- HYAL-1:
-
Hyaluronidase 1
- HAS2:
-
Hyaluronan synthase 2
- HAbp:
-
HA-binding protein
References
Asplund T, Versnel MA, Laurent TC, Heldin P (1993) Human mesothelioma cells produce factors that stimulate the production of hyaluronan by mesothelial cells and fibroblasts. Cancer Res 53:388–392
Auvinen P, Tammi R, Parkkinen J, Tammi M, Agren U, Johansson R, Hirvikoski P, Eskelinen M, Kosma VM (2000) Hyaluronan in peritumoral stroma and malignant cells associates with breast cancer spreading and predicts survival. Am J Pathol 156:529–536. doi:10.1016/S0002-9440(10)64757-8
Csoka AB, Frost GI, Stern R (2001) The six hyaluronidase-like genes in the human and mouse genomes. Matrix Biol 20:499–508
Frisk G, Svensson T, Backlund LM, Lidbrink E, Blomqvist P, Smedby KE (2012) Incidence and time trends of brain metastases admissions among breast cancer patients in Sweden. Br J Cancer 106:1850–1853. doi:10.1038/bjc.2012.163
Fuhrmann IK, Steinhagen J, Ruther W, Schumacher U (2015) Comparative immunohistochemical evaluation of the zonal distribution of extracellular matrix and inflammation markers in human meniscus in osteoarthritis and rheumatoid arthritis. Acta Histochem 117:243–254. doi:10.1016/j.acthis.2014.12.009
Fujisaki T, Tanaka Y, Fujii K, Mine S, Saito K, Yamada S, Yamashita U, Irimura T, Eto S (1999) CD44 stimulation induces integrin-mediated adhesion of colon cancer cell lines to endothelial cells by up-regulation of integrins and c-Met and activation of integrins. Cancer Res 59:4427–4434
Hagenfeld D, Kathagen N, Prehm P (2014) Adsorption of glycosaminoglycans to the cell surface is responsible for cellular donnan effects. J Cell Biochem 115:1334–1341. doi:10.1002/jcb.24791
Hunt LC, Gorman C, Kintakas C, McCulloch DR, Mackie EJ, White JD (2013) Hyaluronan synthesis and myogenesis: a requirement for hyaluronan synthesis during myogenic differentiation independent of pericellular matrix formation. J Biol Chem 288:13006–13021. doi:10.1074/jbc.M113.453209
Itano N, Sawai T, Yoshida M, Lenas P, Yamada Y, Imagawa M, Shinomura T, Hamaguchi M, Yoshida Y, Ohnuki Y, Miyauchi S, Spicer AP, McDonald JA, Kimata K (1999) Three isoforms of mammalian hyaluronan synthases have distinct enzymatic properties. J Biol Chem 274:25085–25092
Knudson W, Biswas C, Toole BP (1984) Interactions between human tumor cells and fibroblasts stimulate hyaluronate synthesis. Proc Natl Acad Sci U S A 81:6767–6771
Kramer MW, Escudero DO, Lokeshwar SD, Golshani R, Ekwenna OO, Acosta K, Merseburger AS, Soloway M, Lokeshwar VB (2011) Association of hyaluronic acid family members (HAS1, HAS2, and HYAL-1) with bladder cancer diagnosis and prognosis. Cancer 117:1197–1209. doi:10.1002/cncr.25565
Lin NU, Claus E, Sohl J, Razzak AR, Arnaout A, Winer EP (2008) Sites of distant recurrence and clinical outcomes in patients with metastatic triple-negative breast cancer: high incidence of central nervous system metastases. Cancer 113:2638–2645. doi:10.1002/cncr.23930
Lokeshwar VB, Cerwinka WH, Isoyama T, Lokeshwar BL (2005) HYAL1 hyaluronidase in prostate cancer: a tumor promoter and suppressor. Cancer Res 65:7782–7789. doi:10.1158/0008-5472.CAN-05-1022
Lu P, Weaver VM, Werb Z (2012) The extracellular matrix: a dynamic niche in cancer progression. J Cell Biol 196:395–406. doi:10.1083/jcb.201102147
McAtee CO, Barycki JJ, Simpson MA (2014) Chapter one—emerging roles for hyaluronidase in cancer metastasis and therapy. In: Melanie AS, Paraskevi H (eds) Advances in cancer research. Academic Press, Cambridge, pp 1–34
Milde-Langosch K, Karn T, Schmidt M, Zu Eulenburg C, Oliveira-Ferrer L, Wirtz RM, Schumacher U, Witzel I, Schutze D, Muller V (2014) Prognostic relevance of glycosylation-associated genes in breast cancer. Breast Cancer Res Treat 145:295–305. doi:10.1007/s10549-014-2949-z
Milde-Langosch K, Schutze D, Oliveira-Ferrer L, Wikman H, Muller V, Lebok P, Pantel K, Schroder C, Witzel I, Schumacher U (2015) Relevance of betaGal-betaGalNAc-containing glycans and the enzymes involved in their synthesis for invasion and survival in breast cancer patients. Breast Cancer Res Treat 151:515–528. doi:10.1007/s10549-015-3425-0
Oskarsson T (2013) Extracellular matrix components in breast cancer progression and metastasis. Breast 22(Suppl 2):S66–S72. doi:10.1016/j.breast.2013.07.012
Posey JT, Soloway MS, Ekici S, Sofer M, Civantos F, Duncan RC, Lokeshwar VB (2003) Evaluation of the prognostic potential of hyaluronic acid and hyaluronidase (HYAL1) for prostate cancer. Cancer Res 63:2638–2644
Prehm P (1984) Hyaluronate is synthesized at plasma membranes. Biochem J 220:597–600
Pukrop T, Dehghani F, Chuang HN, Lohaus R, Bayanga K, Heermann S, Regen T, Van Rossum D, Klemm F, Schulz M, Siam L, Hoffmann A, Trumper L, Stadelmann C, Bechmann I, Hanisch UK, Binder C (2010) Microglia promote colonization of brain tissue by breast cancer cells in a Wnt-dependent way. Glia 58:1477–1489. doi:10.1002/glia.21022
Schmaus A, Sleeman JP (2015) Hyaluronidase-1 expression promotes lung metastasis in syngeneic mouse tumor models without affecting accumulation of small hyaluronan oligosaccharides in tumor interstitial fluid. Glycobiology 25:258–268. doi:10.1093/glycob/cwu106
Schwertfeger KL, Cowman MK, Telmer PG, Turley EA, McCarthy JB (2015) Hyaluronan, inflammation, and breast cancer progression. Front Immunol 6:236. doi:10.3389/fimmu.2015.00236
Sierra A, Price JE, Garcia-Ramirez M, Mendez O, Lopez L, Fabra A (1997) Astrocyte-derived cytokines contribute to the metastatic brain specificity of breast cancer cells. Lab Invest 77:357–368
Simpson MA, Reiland J, Burger SR, Furcht LT, Spicer AP, Oegema TR Jr, McCarthy JB (2001) Hyaluronan synthase elevation in metastatic prostate carcinoma cells correlates with hyaluronan surface retention, a prerequisite for rapid adhesion to bone marrow endothelial cells. J Biol Chem 276:17949–17957. doi:10.1074/jbc.M010064200
Singleton PA (2014) Hyaluronan regulation of endothelial barrier function in cancer. Adv Cancer Res 123:191–209. doi:10.1016/B978-0-12-800092-2.00007-1
Sironen RK, Tammi M, Tammi R, Auvinen PK, Anttila M, Kosma VM (2011) Hyaluronan in human malignancies. Exp Cell Res 317:383–391. doi:10.1016/j.yexcr.2010.11.017
Tammi RH, Kultti A, Kosma VM, Pirinen R, Auvinen P, Tammi MI (2008) Hyaluronan in human tumors: pathobiological and prognostic messages from cell-associated and stromal hyaluronan. Semin Cancer Biol 18:288–295. doi:10.1016/j.semcancer.2008.03.005
Tan JX, Wang XY, Li HY, Su XL, Wang L, Ran L, Zheng K, Ren GS (2011) HYAL1 overexpression is correlated with the malignant behavior of human breast cancer. Int J Cancer 128:1303–1315. doi:10.1002/ijc.25460
Tan JX, Wang XY, Su XL, Li HY, Shi Y, Wang L, Ren GS (2011) Upregulation of HYAL1 expression in breast cancer promoted tumor cell proliferation, migration, invasion and angiogenesis. PLoS ONE 6:e22836. doi:10.1371/journal.pone.0022836
Toole BP (2004) Hyaluronan: from extracellular glue to pericellular cue. Nat Rev Cancer 4:528–539. doi:10.1038/nrc1391
Udabage L, Brownlee GR, Nilsson SK, Brown TJ (2005) The over-expression of HAS2, Hyal-2 and CD44 is implicated in the invasiveness of breast cancer. Exp Cell Res 310:205–217. doi:10.1016/j.yexcr.2005.07.026
Witzel I, Oliveira-Ferrer L, Pantel K, Muller V, Wikman H (2016) Breast cancer brain metastases: biology and new clinical perspectives. Breast Cancer Res 18:8. doi:10.1186/s13058-015-0665-1
Wu M, Cao M, He Y, Liu Y, Yang C, Du Y, Wang W, Gao F (2015) A novel role of low molecular weight hyaluronan in breast cancer metastasis. FASEB J 29:1290–1298. doi:10.1096/fj.14-259978
Acknowledgments
We are grateful for the excellent technical assistance of Maila Rossberg and Kathrin Eylmann.
Funding
This work was funded by the Hamburger Krebsgesellschaft (Grant Number HKG 4048).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Isabell Witzel and Anna K. Marx have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10549_2017_4135_MOESM1_ESM.pptx
Supplementary material 1 (PPTX 1096 kb)
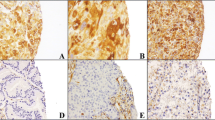
Fig. 1S: HA-binding protein staining pattern of tumor and stroma in breast cancer metastases of various locations. (A) Brain metastasis with HA-binding protein negative to positive tumor-associated staining (T), HA-binding protein negative to positive tumor microenviroment (TME) and HA-binding protein negative to positive blood vessels (BV) (20x, IRS 0-8). (B) Brain metastasis with HA-binding protein positive tumor cells (T) and HA-binding protein positive TME. All tumor cells show HA-binding protein expression in the membrane and some in the cytoplasm (20 × , IRS 2-8 and IRS 6-10). (C) Bone metastasis with HA-binding protein negative tumor cells (T), HA-binding protein positive TME, and HA-binding protein positive blood vessels (BV) (20 × , IRS 0 and IRS 11-12). (D) Skin metastasis with HA-binding protein positive tumor cells (T) and HA-binding protein positive adipocytes (A) (20 × IRS 4-8 and IRS 10-12). (E) Liver metastasis with HA-binding protein negative and weakly stained tumor cells (T) and HA-binding protein positive TME (20 × , IRS 0-4, and 10-12). (F) Lung metastasis with HA-binding protein negative to positive tumor cells (T) and HA-binding protein positive TME (20 × , IRS 0-4 and IRS 8-12)
Rights and permissions
About this article
Cite this article
Witzel, I., Marx, A.K., Müller, V. et al. Role of HYAL1 expression in primary breast cancer in the formation of brain metastases. Breast Cancer Res Treat 162, 427–438 (2017). https://doi.org/10.1007/s10549-017-4135-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-017-4135-6