Abstract
This study aimed to compare the efficacy and safety aspects of three anthracycline-based regimens as neoadjuvant chemotherapy in primary breast cancer. Five-hundred and one patients with clinical stage I–III invasive breast cancer were randomly assigned to receive four cycles of neoadjuvant chemotherapy with either CEFci arm (5-Fu 200 mg/m2 daily by 24-h continuous infusion and epirubicin 100 mg/m2 and cyclophosphamide 600 mg/m2 intravenous bolus on day 1), CEF arm (cyclophosphamide 600 mg/m2, epirubicin 100 mg/m2, and 5-Fu 600 mg/m2 i.v. on day 1), or EC arm (epirubicin 100 mg/m2 and cyclophosphamide 600 mg/m2 i.v. on day 1). The pathologic responses to chemotherapy were assessed according to the Miller and Payne grading system (MP). A total of 485 patients were included in the intent-to-treat population. Breast pathologic complete response (pCR) rate was 18.9 % (31/164) in CEFci arm, 15.0 % (24/160) in CEF arm, and 12.4 % (20/161) in EC arm (P = 0.266). MP grading system 4/5 response rate was significantly higher in CEFci arm than that in CEF arm and EC arm (44.5, 31.3 and 27.3 %, respectively, P = 0.003). There was no significant difference on grade III/IV neutropenia among three arms (P = 0.538), but thrombocytopenia, decreased hemoglobin, and elevated aminotransferase appeared to be observed more in CEFci arm (P = 0.040, 0.059, and 0.073, respectively). CEFci did not reach a higher pCR rate compared with CEF or EC in patients with primary breast cancer. The potential advantage of CEFci in improving pathologic response still requires further research. The accompanied hematologic and biochemical toxicities, and the catheter-related complications should also be noted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neoadjuvant chemotherapy is the standard treatment for locally advanced and inflammatory breast cancer and is applied to downstage tumors initially not suitable for resection or breast-conserving surgery [1, 2]. Moreover, the application of neoadjuvant chemotherapy in operable breast cancer is gradually increasing because of the advantages of using the tumor as an early in vivo measure of response to treatment [3]. Previous studies showed that tumor response to neoadjuvant chemotherapy was associated with prognosis of breast cancer patients, and those who achieved the pathologic complete response (pCR) potentially obtain better survival [4, 5]. Currently, anthracycline-based polychemotherapy regimens are frequently used in the adjuvant treatment in breast cancer, and 5-fluorouracil (5-Fu) is also commonly included [6–8]. In contrast with intravenous bolus 5-Fu, continuous infusion of 5-Fu showed a more significant efficacy and less adverse reactions with a relatively short half-life (<30 min) [9, 10]. In order to search for a more effective regimen of 5-Fu combined with epirubicin and cyclophosphamide, we designed a single-center, randomized, parallel controlled study to compare the efficacy and safety aspects of three different regimens: cyclophosphamide/epirubicin/intravenous bolus 5-Fu (CEF), cyclophosphamide/epirubicin/continuous infusional 5-Fu (CEFci), and cyclophosphamide/epirubicin (EC) with absence of 5-Fu.
Methods
Patients
We included in the study female clinical stage I–III (cT1–3N0–2M0) breast cancer patients aged ≤65 years old with histologically confirmed invasive breast cancer by core needle biopsy in our breast center from March 2011 to July 2014. All subtypes defined by immunohistochemistry hormone receptor (HR) and human epidermal growth factor receptor 2 (HER2) status were enrolled in the study, including HR-positive and HER2-negative subtype (HR+HER2−), triple-negative subtype (HR−HER2−), and HER2-positive subtype (HER2+). Further relevant eligibility criteria included no history of other malignancies; adequate hematologic function (absolute neutrophils count ≥1.5 × 109/L, platelets ≥100 × 109/L, and hemoglobin ≥100 g/L); adequate hepatic and renal functions [alanine aminotransferase (ALT)/aspartate aminotransferase (AST) ≤2.5 times the institutional upper normal limit (UNL), serum bilirubin ≤1.5 × UNL, and serum creatinine ≤1.7 mg/dl]; and normal cardiac function. Patients with pregnancy or in lactation; with known or suspected distant metastases diagnosed by chest X-ray, abdominal and pelvic ultrasound, or bone scans; or with currently uncontrolled diseases (e.g., cardiac dysrhythmias, unstable diabetes) or active infection were excluded. Patients with the Miller and Payne (MP) grading results were included in the intention-to-treat analyses (ITT), and those without neoadjuvant chemotherapy or without surgery were excluded. All the enrolled patients were provided with written informed consent.
Study design
This is a single-center, randomized, and parallel controlled study designed to compare the efficacy and safety aspects among three anthracycline-based regimens for neoadjuvant chemotherapy in patients with primary breast cancer, registered with ClinicalTrials.gov, number NCT01199432.
Patients were centrally randomly assigned (1:1:1) to receive one of the three neoadjuvant chemotherapy: Arm A: cyclophosphamide, epirubicin, and continuous infusional 5-fluorouracil (CEFci); Arm B: cyclophosphamide, epirubicin, and intravenous bolus 5-fluorouracil (CEF); or Arm C: cyclophosphamide and epirubicin (EC). After completion of four cycles of neoadjuvant chemotherapy, patients underwent surgery and possible following adjuvant treatment (chemotherapy, trastuzumab, radiotherapy, or endocrinotherapy) according to the guidelines of NCCN and St. Gallen [11].
Treatment
All eligible patients received cyclophosphamide (C, 600 mg/m2) plus epirubicin (E, 100 mg/m2) administered as intravenous boluses on day 1 of every 3 weeks. In arm A (CEFci regimen), 5-fluorouracil (5-Fu, 200 mg/m2) was administered as a 24-h continuous infusion using an ambulatory pump via Hickman line through a central venous catheter for 12 weeks; in arm B (CEF regimen), 5-Fu (600 mg/m2) was administered as intravenous bolus on day 1 every 3 weeks; however, in arm C (EC regimen), 5-Fu was not included.
Toxicity and chemotherapy delay
Toxicity was assessed according to common toxicity criteria grade by laboratory examination, including routine blood tests on day 7, 10, 14, 21 and biochemistry test on day 21 before every next cycle of chemotherapy. Recombinant human granulocyte colony-stimulating factor was applied at 5 µg/kg/d subcutaneously for three days if leukocyte count <2.0 × 109/L and/or absolute neutrophils count <1.0 × 109/L, and antibiotics were prescribed simultaneously for any episode of febrile neutropenia. Recombinant human interleukin-11 support was given at 24 million IU/d subcutaneously for seven days if platelets count <50 × 109/L, and liver-protection therapy was conducted if ALT/AST level was found higher than 2.5 times the institutional UNL.
Treatment was delayed for one or two weeks when absolute neutrophils count <1.5 × 109/L, platelets count <100 × 109/L, hemoglobin count <80 g/L, ALT/AST ≥ 2.5 × UNL, and/or when blood urea nitrogen/creatinine was found abnormal during hematologic and biochemical tests on day 21 before every next cycle of chemotherapy. We terminated neoadjuvant chemotherapy if treatment was delayed for more than two weeks, if there was incidence of disease progression or severer adverse events, or according to the patient’s wills.
Response assessment
Tumor clinical response to chemotherapy was both assessed by ultrasonography after completion of the second and the fourth cycles of treatment before surgery using the World Health Organization criteria. Complete response (CR) was defined as complete resolution of all masses and abnormalities. Partial response (PR) was defined as a ≥50 % reduction in the product of the bidimensional tumor measurements without progression of any lesion or appearance of any new disease. For stable disease (SD), there was a <50 % reduction or <25 % increase, whereas for progressive disease (PD), there was a ≥25 % increase or appearance of new disease [12].
Tissue slices from core needle biopsy both before neoadjuvant chemotherapy and surgery were collected from the Department of Pathology, and the histologic response to chemotherapy in breast was assessed by two senior pathologists using the Miller and Payne grading system: Grade 1 (G1): no change or some alteration to individual malignant cells but no reduction in overall cellularity; Grade 2 (G2): a minor loss of tumor cells but overall cellularity still high—up to 30 % loss; Grade 3 (G3): between an estimated 30 and 90 % reductions in tumor cells; Grade 4 (G4): a marked disappearance of tumor cells such that only small clusters or widely dispersed individual cells remain—more than 90 % loss of tumor cells; and Grade 5 (G5): no malignant cells identifiable in sections from the site of the tumor—only vascular fibroelastotic stroma remains often containing macrophages. However, ductal carcinoma in situ may be present [13]. Each pathologist scored the tumor tissues independently, and agreement by consensus was achieved if necessary. In this study, we defined grade 5 (G5) as pCR [14].
In this study, the primary end point was pathologic complete response, and the secondary endpoints were MP response, clinical response, and adverse effect.
Sample size calculation and statistics
Based on an expected rate of pathologic complete response with 10 % in arm C (EC regimen), 25 % in arm B (CEF regimen), and 40 % in arm A (CEFci regimen), 456 patients were calculated as a minimum sample size to provide 80 % power, with α = 0.05 (two-sided) level of significance to detect an absolute difference in pCR rate in excess of 15 % among arms. With possible follow-up loss of less than 10 % of patients, the total sample size was determined as 501.
Number or percentage was used to describe categorical variables. The comparison of pCR rate or clinicopathologic characteristics among the arms was performed with Chi square test or Fisher’s exact test. Logistic regression model was carried out to compare the odds ratios (ORs) for pathologic response among the arms in multivariate analyses. All statistical tests were two-sided, and bonferroni correction was used for multiple comparisons. P values < 0.05 were considered statistically significant.
Sample size calculation and statistical analyses were performed using PASS 2008 software and SPSS 17.0 software, respectively.
Results
Patients
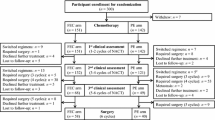
Between March 11, 2011 and July 17, 2014, a total of 501 patients (167 assigned to each arm) were enrolled from the Breast Center, Peking University Cancer Hospital. As Table 1 shows, characteristics (including age, menstrual status, tumor size, lymph node status, pathological type, histologic grade, IHC-defined subtype, ki-67 index, and surgery type) were balanced across the three treatment arms. Four (two in arm B and two in arm C) patients were found to be ineligible after randomization, leaving 497 patients in total receiving neoadjuvant chemotherapy. Of the four ineligible patients, three underwent surgery immediately due to uncontrolled hypertension, severe anemia, and thrombocytopenia, respectively, and the remaining one refused any treatment. Twelve (3 in arm A, 5 in arm B, and 4 in arm C) of 497 patients did not undergo surgery due to their being lost to follow-up, leaving 485 with available pathologic response to chemotherapy in breast for the ITT analyses. Of these 485 patients, the following patients were omitted: 10 (9 in arm A and 1 in arm C) who were against protocols and selected regimens independently after randomization; and 10 (all in arm A) who had terminated continuous infusional 5-fluorouracil through central venous catheters after completion of one cycle of neoadjuvant chemotherapy (six with catheter thrombus, one with catheter infection, and three since they refused). Neoadjuvant chemotherapy was discontinued on 14 patients (7 in arm A, 4 in arm B, and 3 in arm C) due to severe adverse events, including febrile neutropenia, hepatic injury, nausea and vomiting, oral ulcer, epilepsy, atrial fibrillation, and palpitation. According to tumor response and/or patient’s wills, neoadjuvant treatment was adjusted for surgery, endocrinotherapy, or followed by four cycles of paclitaxel regimen on 28 patients (3 in arm A, 12 in arm B, and 13 in arm C) (Fig. 1).
Efficacy
The primary analyses of tumor response to neoadjuvant chemotherapy were carried out in the intention-to-treat population of 485 patients: 164 (33.8 %) in arm A, 160 (32.9 %) in arm B, and 161 (33.2 %) in arm C (Table 2). Of these 485 patients, a total of 75 (15.5 %) achieved the pCR in breast, and there were no statistically significant differences in pCR rates among the three arms (18.9 vs. 15.0, and 12.4 %, P = 0.266) (Table 2). The percentages of patients with clinical CR or PR was 63.4 % (104/164) in arm A, 53.8 % (86/160) in arm B, and 52.8 % (85/161) in arm C, with no statistically significant differences (P = 0.101) (Table 2). As Table 3 shows, the percentage of patients with MP 4/5 response in breast was higher in arm A than that in arm B (44.5 vs. 31.3 %) with statistically significant difference (P = 0.004), and an OR of 2.081 (95 % CI 1.264–3.427), but the percentage in arm B was similar to that in arm C (31.3 vs. 27.3 %), with no statistically significant difference (OR 0.821, 95 % CI 0.489–1.378, P = 0.455). Besides treatment regimens, pathologic response to neoadjuvant chemotherapy in breast was also associated with tumor size, ki-67 index, and IHC-defined subtype.
Hematologic and biochemical toxicity
The percentages of patients with grade III/IV neutropenia were 80.5 % (132/164) in arm A, 74.4 % (119/160) in arm B, and 72.0 % (116/161) in arm C, respectively, and there was no statistically significant difference among the three groups (P = 0.538) (Table 4). Hemoglobin count ≤ 109 g/L (grade I–IV) was observed in 56 (34.1 %) of 164 patients in arm A, 48 (30.0 %) of 160 patients in arm B, and 36 (22.4 %) of 161 patients in arm C, and the difference was close to statistical significance (P = 0.059). The percentages of patients with thrombocytopenia were 9.1 % (15/164) in arm A, including 1.2 % with grade III/IV; 2.5 % (4/160) in arm B; and 7.5 % (12/161) in arm C, and the difference was statistically significant (P = 0.040). The percentages of patients with ALT/AST > 2.5 times the institutional UNL were 9.1 % (15/164) in arm A, 4.4 % (7/160) in arm B, and 3.7 % (6/161) in arm C, respectively, and the difference was close to statistical significance (P = 0.073).
Discussion
Clinically, 5-fluorouracil (5-Fu) has been commonly used as a single agent or in combination with other chemotherapies in breast cancer treatment for decades [15–17]. Continuous infusional 5-Fu combined with epirubicin and cisplatin (ECciF) regimen appears to be more active than conventional regimens for both advanced breast cancer and large operable breast cancer, but the contribution of 5-Fu to the anthracycline–cyclophosphamide regimens (AC or EC) has not been well defined [18, 19].
Our study showed that the outcome of administration of low-dose continuous infusional 5-Fu (200 mg/m2) 24 h daily for 21 days was not superior to that of high-dose intravenous bolus (600 mg/m2) on day 1, with no statistically significant difference in pathologic complete responses in breast between CEFci arm and CEF arm, although the percentage of patients achieving MP 4/5 response in breast in CEFci arm was almost as twice as that in CEF arm. Compared to the treatment without 5-Fu in the regimen, 5-Fu administrated as intravenous bolus 600 mg/m2 on day 1 did not show any significant advantage of efficacy improvement, with MP 4/5 response rate of 31.3 % in CEF arm versus 27.3 % in EC arm. In the TOPIC trial which randomly designed 426 patients with operable breast cancer tumor ≥3 cm to receive six cycles of either epirubicin–cisplatin and continuous infusional 5-Fu (infusional ECisF) or doxorubicin–cyclophosphamide (AC) before surgery to compare the response rates between the two arms [20], no statistically significant differences were observed both in overall response rates (77 vs. 75 %, P = 0.6) and in the pCR rates (both 16 %, P = 1.0). The discrepant results between our study and those of the TOPIC trial possibly arise from the different chemotherapeutic agent doses and regimens employed.
In the aspect of hematologic toxicity, no statistically significant differences were observed in the incidences of grade III/IV neutropenia among the three arms in our study (80.5, 74.4, and 72.0 %, respectively, P = 0.538), but thrombocytopenia and decreased hemoglobin appeared to be observed more in CEFci arm compared with the other two arms, with differences being statistically significant or close to statistical significance (P = 0.040,and 0.059, respectively). In terms of biochemical toxicity, however, elevated aminotransferase occurred in 9.1 % of patients receiving CEFci regimen, which is higher than 4.4 % with CEF regimen and 3.7 % with EC regimen, and the difference was close to statistical significance (P = 0.073).
It is noteworthy that nine patients in CEFci arm rejected the protocols after randomization and before the beginning of the first cycle of chemotherapy, since they were worried that their daily life would be influenced by probable inconvenience of continuous infusional 5-Fu and periodic maintenance of central venous catheters. Similarly, three patients refused continuous infusional 5-Fu during the process of treatment, and ci 5-Fu was ceased in another seven patients after the removal of catheters due to catheter-related events, such as thrombus and infection. However, treatment plans were changed apparently more frequently in patients receiving EC or CEF regimen according to tumor response assessed by ultrasonography after completion of two or four cycles of neoadjuvant chemotherapy, and 25 patients with SD or PD received either four cycles of paclitaxel chemotherapy or endocrinotherapy, or underwent surgery immediately according to physician’s decisions and/or patient’s wills.
To our knowledge, this is the only randomized controlled clinical study comparing the efficacy and safety aspects of different anthracycline-based regimens in primary breast cancer neoadjuvant chemotherapy between standard epirubicin-cyclophosphamide (EC) regimen and EC combined with 5-Fu, as well as between continuous infusion and intravenous bolus as two different administration approaches of 5-Fu.
In conclusion, although this study shows that there are no statistically significant differences in pCR rates among the three arms, the potential advantage of the administration of low-dose continuous infusional 5-Fu in improving the efficacy of anthracycline-based chemotherapy still requires further research. However, the accompanied hematologic and biochemical toxicities, catheter-related complications, and poor compliance with inconvenient daily life should also be noted in patients receiving the CEFci regimen.
References
Iqbal J, Shafi AA, Alharthi BN (2014) Neoadjuvant chemotherapy in locally advanced breast cancer. J Coll Phys Surg Pak 24(11):845–848
Caudle AS, Kuerer HM (2014) Breast conservation therapy after neoadjuvant chemotherapy: optimization of a multimodality approach. J Surg Oncol 110(1):32–36
Read RL, Flitcroft K, Snook KL, Boyle FM, Spillane AJ (2015) Utility of neoadjuvant chemotherapy in the treatment of operable breast cancer. ANZ J Surg 85(5):315–320
Berruti A, Amoroso V, Gallo F, Bertaglia V, Simoncini E, Pedersini R, Ferrari L, Bottini A, Bruzzi P, Sormani MP (2014) Pathologic complete response as a potential surrogate for the clinical outcome in patients with breast cancer after neoadjuvant therapy: a meta-regression of 29 randomized prospective studies. J Clin Oncol 32(34):3883–3891
Wang-Lopez Q, Chalabi N, Abrial C, Radosevic-Robin N, Durando X, Mouret-Reynier MA, Benmammar KE, Kullab S, Bahadoor M, Chollet P et al (2015) Can pathologic complete response (pCR) be used as a surrogate marker of survival after neoadjuvant therapy for breast cancer? Crit Rev Oncol Hematol 95(1):88–104
Adlard JW, Dodwell DJ (2001) Optimum anthracycline-based chemotherapy for early breast cancer. Lancet Oncol 2(8):469–474
Sirohi B, A’Hern R, Coombes G, Bliss JM, Hickish T, Perren T, Crawford M, O’Brien M, Iveson T, Ebbs S et al (2010) A randomised comparative trial of infusional ECisF versus conventional FEC as adjuvant chemotherapy in early breast cancer: the TRAFIC trial. Ann Oncol 21(8):1623–1629
Del Mastro L, De Placido S, Bruzzi P, De Laurentiis M, Boni C, Cavazzini G, Durando A, Turletti A, Nisticò C, Valle E et al (2015) Fluorouracil and dose-dense chemotherapy in adjuvant treatment of patients with early-stage breast cancer: an open-label, 2 × 2 factorial, randomised phase 3 trial. Lancet 385(9980):1863–1872
Nolè F, Munzone E, Mandalà M, Catania C, Orlando L, Zampino MG, Minchella I, Colleoni M, Peruzzotti G, Marrocco E et al (2001) Vinorelbine, cisplatin and continuous infusion of 5-fluorouracil (ViFuP) in metastatic breast cancer patients: a phase II study. Ann Oncol 12(1):95–100
Regierer AC, Reinecke F, Weigel A, Dieing A, Lehenbauer-Dehm S, Schwarzlose-Schwarck S, Possinger K, Eucker J (2011) 5FU continuous infusion in heavily pretreated advanced breast cancer patients. Onkologie 34(12):696–700
Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thürlimann B, Senn HJ, Panel members (2011) Strategies for subtypes-dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer. Ann Oncol 22(8):1736–1747
Yang WT, Dryden MJ, Gwyn K, Whitman GJ, Theriault R (2006) Imaging of breast cancer diagnosed and treated with chemotherapy during pregnancy. Radiology 239(1):52–60
Ogston KN, Miller ID, Payne S, Hutcheon AW, Sarkar TK, Smith I, Schofield A, Heys SD (2003) A new histological grading system to assess response of breast cancers to primary chemotherapy: prognostic significance and survival. Breast 12(5):320–327
Qi M, Li JF, Xie YT, Lu AP, Lin BY, Ouyang T (2010) Weekly paclitaxel improved pathologic response of primary chemotherapy compared with standard 3 weeks schedule in primary breast cancer. Breast Cancer Res Treat 123(1):197–202
Smith IE (1996) Continuous infusional 5-fluorouracil in breast cancer or the revival of an old drug? Ann Oncol 7(8):771–772
O’Byrne KJ, Koukourakis MI, Saunders MP, Salisbury AJ, Isaacs R, Varcoe S, Taylor M, Ganesan TS, Harris AL, Talbot DC (1998) Cyclophosphamide, methotrexate and infusional 5-fluorouracil (infusional CMF) in metastatic breast cancer. Br J Cancer 77(11):1950–1956
Stuart NS, Mclllmurray MB, Bishop JL, Johnston SR, Price CG, O’Reilly SM, Joffe JK, Neave F, Whipp EC (2008) Vinorelbine and infusional 5-fluorouracil in anthracycline and taxane pre-treated metastatic breast cancer. R Coll Radiol 20(2):152–156
Smith IE, Walsh G, Jones A, Prendiville J, Johnston S, Gusterson B, Ramage F, Robertshaw H, Sacks N, Ebbs S et al (1995) High complete remission rates with primary neoadjuvant infusional chemotherapy for large early breast cancer. J Clin Oncol 13(2):424–429
Jones AL, Smith IE, O’Brien ME, Talbot D, Walsh G, Ramage F, Robertshaw H, Ashley S (1994) Phase II study of continuous infusion fluorouracil with epirubicin and cisplatin in patients with metastatic and locally advanced breast cancer: an active new regimen. J Clin Oncol 12(6):1259–1265
Smith IE, A’Hern RP, Coombes GA, Howell A, Ebbs SR, Hickish TF, O’Brien ME, Mansi JL, Wilson CB, Robinson AC et al (2004) A novel continuous infusional 5-fluorouracil-based chemotherapy regimen compared with conventional chemotherapy in the neo-adjuvant treatment of early breast cancer: 5 year results of the TOPIC trial. Ann Oncol 15(5):751–758
Acknowledgments
The authors would like to acknowledge all the nurses in the breast center for their services, and women involved in this study.
Funding
This study was supported by graunts from Beijing Science & technique committee priority project (D09050703570904)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standard
This study complies with current Chinese law.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Zhou, Y., Ouyang, T., Xie, Y. et al. A single-center, randomized, parallel controlled study comparing the efficacy and safety aspects of three anthracycline-based regimens as neoadjuvant chemotherapy in primary breast cancer. Breast Cancer Res Treat 157, 527–534 (2016). https://doi.org/10.1007/s10549-016-3843-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-016-3843-7