Abstract
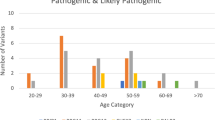
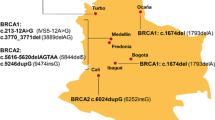
We evaluated the clinical utility of screening for mutations in 34 breast/ovarian cancer susceptibility genes in high-risk families in Israel. Participants were recruited from 12, 2012 to 6, 2015 from 8 medical centers. All participants had high breast/ovarian cancer risk based on personal and family history. Genotyping was performed with the InVitae™ platform. The study was approved by the ethics committees of the participating centers; all participants gave a written informed consent before entering the study. Overall, 282 individuals participated in the study: 149 (53 %) of Ashkenazi descent, 80 (28 %) Jewish non-Ashkenazi descent, 22 (8 %) of mixed Ashkenazi/non-Ashkenazi origin, 21 (7 %) were non-Jewish Caucasians, and the remaining patients (n = 10–3.5 %) were of Christian Arabs/Druze/unknown ethnicity. For breast cancer patients (n = 165), the median (range) age at diagnosis was 46 (22–90) years and for ovarian cancer (n = 15) 54 (38–69) years. Overall, 30 cases (10.6 %) were found to carry a pathogenic actionable mutation in the tested genes: 10 BRCA1 (3 non-founder mutations), 9 BRCA2 (8 non-founder mutations), and one each in the RAD51C and CHEK2 genes. Furthermore, actionable mutations were detected in 9 more cases in 4 additional genes (MSH2, RET, MSH6, and APC). No pathogenic mutations were detected in the other genotyped genes. In this high-risk population, 10.6 % harbored an actionable pathogenic mutation, including non-founder mutations in BRCA1/2 and in additional cancer susceptibility genes, suggesting that high-risk families should be genotyped and be assigned a genotype-based cancer risk.
Similar content being viewed by others
References
Petrucelli N, Daly MB, Feldman GL (2010) Hereditary breast and ovarian cancer due to mutations in BRCA1 and BRCA2. Genet Med 12:245–259
Abeliovich D, Kaduri L, Lerer I, Weinberg N, Amir G, Sagi M, Zlotogora J, Heching N, Peretz T (1997) The founder mutations 185delAG and 5382insC in BRCA1 and 6174delT in BRCA2 appear in 60% of ovarian cancer and 30% of early-onset breast cancer patients among Ashkenazi women. Am J Hum Genet 60:505–514
Tobias DH, Eng C, McCurdy LD, Kalir T, Mandelli J, Dottino PR, Cohen CJ (2000) Founder BRCA 1 and 2 mutations among a consecutive series of Ashkenazi Jewish ovarian cancer patients. Gynecol Oncol 78:148–151
Warner E, Foulkes W, Goodwin P, Meschino W, Blondal J, Paterson C, Ozcelik H, Goss P, Allingham-Hawkins D, Hamel N, Di Prospero L, Contiga V, Serruya C, Klein M, Moslehi R, Honeyford J, Liede A, Glendon G, Brunet JS, Narod S (1999) Prevalence and penetrance of BRCA1 and BRCA2 gene mutations in unselected Ashkenazi Jewish women with breast cancer. J Natl Cancer Inst 91:1241–1247
Hartge P, Struewing JP, Wacholder S, Brody LC, Tucker MA (1999) The prevalence of common BRCA1 and BRCA2 mutations among Ashkenazi Jews. Am J Hum Genet 64:963–970
Kauff ND, Perez-Segura P, Robson ME, Scheuer L, Siegel B, Schluger A, Rapaport B, Frank TS, Nafa K, Ellis NA, Parmigiani G, Offit K (2002) Incidence of non-founder BRCA1 and BRCA2 mutations in high risk Ashkenazi breast and ovarian cancer families. J Med Genet 39:611–614
Palma MD, Domchek SM, Stopfer J, Erlichman J, Siegfried JD, Tigges-Cardwell J, Mason BA, Rebbeck TR, Nathanson KL (2008) The relative contribution of point mutations and genomic rearrangements in BRCA1 and BRCA2 in high-risk breast cancer families. Cancer Res 68:7006–7014
Shiri-Sverdlov R, Gershoni-Baruch R, Ichezkel-Hirsch G, Gotlieb WH, Bruchim Bar-Sade R, Chetrit A, Rizel S, Modan B, Friedman E (2001) The Tyr978X BRCA1 mutation in non-Ashkenazi Jews: occurrence in high-risk families, general population and unselected ovarian cancer patients. Community Genet 4:50–55
Lerer I, Wang T, Peretz T, Sagi M, Kaduri L, Orr-Urtreger A, Stadler J, Gutman H, Abeliovich D (1998) The 8765delAG mutation in BRCA2 is common among Jews of Yemenite extraction. Am J Hum Genet 63:272–274
Hilbers FS, Vreeswijk MP, van Asperen CJ, Devilee P (2013) The impact of next generation sequencing on the analysis of breast cancer susceptibility: a role for extremely rare genetic variation? Clin Genet 84:407–414
Catucci I, Milgrom R, Kushnir A, Laitman Y, Paluch-Shimon S, Volorio S, Ficarazzi F, Bernard L, Radice P, Friedman E, Peterlongo P (2012) Germline mutations in BRIP1 and PALB2 in Jewish high cancer risk families. Fam Cancer 11:483–491
Kushnir A, Laitman Y, Shimon SP, Berger R, Friedman E (2012) Germline mutations in RAD51C in Jewish high cancer risk families. Breast Cancer Res Treat 136:869–874
Figer A, Kaplan A, Frydman M, Lev D, Paswell J, Papa MZ, Goldman B, Friedman E (2002) Germline mutations in the PTEN gene in Israeli patients with Bannayan-Riley-Ruvalcaba syndrome and women with familial breast cancer. Clin Genet 62:298–302
Kurian AW, Hare EE, Mills MA, Kingham KE, McPherson L, Whittemore AS, McGuire V, Ladabaum U, Kobayashi Y, Lincoln SE, Cargill M, Ford JM (2014) Clinical evaluation of a multiple-gene sequencing panel for hereditary cancer risk assessment. J Clin Oncol 32:2001–2009
Lincoln SE, Kobayashi Y, Anderson MJ, Yang S, Desmond AJ, Mills MA, Nilsen GB, Jacobs KB, Monzon FA, Kurian AW, Ford JM, Ellisen LW (2015) A Systematic comparison of traditional and multigene panel testing for hereditary breast and ovarian cancer genes in more than 1000 patients. J Mol Diagn 17:533–544
Lynch HT, Watson P, Tinley S, Snyder C, Durham C, Lynch J, Kirnarsky Y, Serova O, Lenoir G, Lerman C, Narod SA (1999) An update on DNA-based BRCA1/BRCA2 genetic counseling in hereditary breast cancer. Cancer Genet Cytogenet 109:91–98
Green RC, Berg JS, Grody WW, Kalia SS, Korf BR, Martin CL, McGuire AL, Nussbaum RL, O’Daniel JM, Ormond KE, Rehm HL, Watson MS, Williams MS, Biesecker LG, College American, American College of Medical Genetics and Genomics (2013) ACMG recommendations for reporting of incidental findings in clinical exome and genome sequencing. Genet Med 15:565–574
Tavtigian SV, Oefner PJ, Babikyan D et al (2009) Rare, evolutionarily unlikely missense substitutions in ATM confer increased risk of breast cancer. Am J Hum Genet 85:427–446
Zhang B, Beeghly-Fadiel A, Long J, Zheng W (2011) Genetic variants associated with breast-cancer risk: comprehensive research synopsis, meta-analysis, and epidemiological evidence. Lancet Oncol 12:477–488
Shen L, Yin ZH, Wan Y, Zhang Y, Li K, Zhou BS (2012) Association between ATM polymorphisms and cancer risk: a meta-analysis. Mol Biol Rep 39:5719–5725
Laitman Y, Simeonov M, Herskovitz L, Kushnir A, Shimon-Paluch S, Kaufman B, Zidan J, Friedman E (2012) Recurrent germline mutations in BRCA1 and BRCA2 genes in high risk families in Israel. Breast Cancer Res Treat 133:1153–1157
Laitman Y, Borsthein RT, Stoppa-Lyonnet D, Dagan E, Castera L, Goislard M, Gershoni-Baruch R, Goldberg H, Kaufman B, Ben-Baruch N, Zidan J, Maray T, Soussan-Gutman L, Friedman E (2011) Germline mutations in BRCA1 and BRCA2 genes in ethnically diverse high risk families in Israel. Breast Cancer Res Treat 127:489–495
Rosenthal E, Moyes K, Arnell C, Evans B, Wenstrup RJ (2015) Incidence of BRCA1 and BRCA2 non-founder mutations in patients of Ashkenazi Jewish ancestry. Breast Cancer Res Treat 149:223–227. Erratum in: Breast Cancer Res Treat (2015) 151:233
Petrucelli N, Mange S, Fulbright JL, Dohany L, Zakalik D, Duquette D (2015) To reflex or not: additional BRCA1/2 testing in Ashkenazi Jewish individuals without founder mutations. J Genet Couns 24:285–293
Pelttari LM, Heikkinen T, Thompson D, Kallioniemi A, Schleutker J, Holli K, Blomqvist C, Aittomäki K, Bützow R, Nevanlinna H (2011) RAD51C is a susceptibility gene for ovarian cancer. Hum Mol Genet 20:3278–3288
Kuusisto KM, Bebel A, Vihinen M, Schleutker J, Sallinen SL (2011) Screening for BRCA1, BRCA2, CHEK2, PALB2, BRIP1, RAD50, and CDH1 mutations in high-risk Finnish BRCA1/2-founder mutation-negative breast and/or ovarian cancer individuals. Breast Cancer Res 13:R20
Walsh T, Casadei S, Coats KH, Swisher E, Stray SM, Higgins J, Roach KC, Mandell J, Lee MK, Ciernikova S, Foretova L, Soucek P, King MC (2006) Spectrum of mutations in BRCA1, BRCA2, CHEK2, and TP53 in families at high risk of breast cancer. JAMA 295:1379–1388
Zick A, Cohen S, Hamburger T, Goldberg Y, Zvi N, Sagi M, Peretz T (2015) A BRCA1 frameshift mutation in women of Kurdish Jewish Descent. Open Breast Cancer J. doi:10.2174/1876817220150529E001
NCCN guidelines. Genetic/familial high-risk assessment: Breast and ovarian. Version 2.2015. http://www.nccn.org/professionals/physician_gls/pdf/genetics_screening.pdf. Accessed 11 Nov 2015
Castéra L, Krieger S, Rousselin A, Legros A, Baumann JJ, Bruet O, Brault B, Fouillet R, Goardon N, Letac O, Baert-Desurmont S, Tinat J, Bera O, Dugast C, Berthet P, Polycarpe F, Layet V, Hardouin A, Frébourg T, Vaur D (2014) Next-generation sequencing for the diagnosis of hereditary breast and ovarian cancer using genomic capture targeting multiple candidate genes. Eur J Hum Genet 22:1305–1313
Tung N, Battelli C, Allen B, Kaldate R, Bhatnagar S, Bowles K, Timms K, Garber JE, Herold C, Ellisen L, Krejdovsky J, DeLeonardis K, Sedgwick K, Soltis K, Roa B, Wenstrup RJ, Hartman AR (2015) Frequency of mutations in individuals with breast cancer referred for BRCA1 and BRCA2 testing using next-generation sequencing with a 25-gene panel. Cancer 121:25–33
Acknowledgments
We thank Dr. Steve Lincoln from InVitae Corporation for his insightful comments. We thank the following clinicians for referring patients: V Libman (Assaf Harofe Medical Center); R Shtoyerman (Kaplan Medical Center), N Siegelmann-Danieli (Maccabi Healthcare Services); B Klein and E Reinstein (Meir Medical Center); N Levi, N Peled, and SM Stemmer (Rabin Medical Center); A Amit, M Ben Harush, K Drumea, R Epelbaum, and M Wollner (Rambam Health Care Campus); A Ben Yehuda, H Friedman, D Kelsen, G Lazer, S Liberman, O Rosengarten, and S Zukerman (Shaare Zedek Medical Center); K Levanon (Sheba Medical Center); N Ashkenazi, F Azem, Dalit Barel, P Kahn, O Merimsky, E Naparstek, S Pelles Avraham, and G Rozner (Sourasky Medical Center); J Zidan and S Mordechai (Ziv Medical Center).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
LSG, YK, AD, and YG are Teva Pharmaceuticals employees. YY is a consultant to Oncotest Teva. EF is an ad hoc consultant to Oncotest Teva.
Additional information
Tamar Yablonski-Peretz and Shani Paluch-Shimon authors have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Yablonski-Peretz, T., Paluch-Shimon, S., Gutman, L.S. et al. Screening for germline mutations in breast/ovarian cancer susceptibility genes in high-risk families in Israel. Breast Cancer Res Treat 155, 133–138 (2016). https://doi.org/10.1007/s10549-015-3662-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-015-3662-2