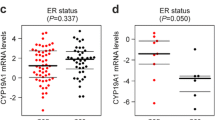
Abstract
To determine whether CYP19A1 polymorphisms are associated with abnormal activity of aromatase and with musculoskeletal and bone side effects of aromatase inhibitors. DNA was isolated from tumor specimens of 4861 postmenopausal women with hormone receptor-positive breast cancer enrolled in the BIG 1–98 trial to receive tamoxifen and/or letrozole for 5 years. Tumors were genotyped for six CYP19A1 polymorphisms using PCR-based methods. Associations with breast cancer-free interval (BCFI), distant recurrence-free interval (DRFI), musculoskeletal and bone adverse events (AEs) were assessed using Cox proportional hazards models. All statistical tests were two-sided. No association between the CYP19A1 genotypes and BCFI or DRFI was observed overall. A reduced risk of a breast cancer event for tamoxifen-treated patients with rs700518 variants was observed (BCFI CC/TC vs. TT: HR 0.53, 95 % CI 0.34–0.82, interaction P = 0.08), but not observed for letrozole-treated patients. There was an increased risk of musculoskeletal AEs for patients with rs700518 variants CC/TC versus TT (HR 1.22, 95 % CI 1.03–1.45, P = 0.02), regardless of treatment. Tamoxifen-treated patients with rs4646 variants had a reduced risk of bone AEs (AA/CA vs. CC: HR 0.76, 95 % CI 0.59–0.98), whereas an increase of minor allele (C) of rs10046 was associated with an increased risk of bone AEs (HR 1.28, 95 % CI 1.07–1.52). rs936308 variants were associated with a reduced risk of bone AEs in letrozole-treated patients (GG/GC vs. CC: HR 0.73, 95 % CI 0.54–0.99), different from in tamoxifen-treated patients (GG/GC vs. CC: HR 1.32, 95 % CI 0.92–1.90, interaction P = 0.01). CYP19A1 rs700518 variants showed associations with BCFI, DRFI, in tamoxifen treated patients and musculoskeletal AEs regardless of treatment. SNPs rs4646, rs10046, and rs936308 were associated with bone AEs.




Similar content being viewed by others
References
Ma CX, Adjei AA, Salavaggione OE, Coronel J, Pelleymounter L, Wang L, Eckloff BW, Schaid D, Wieben ED, Adjei AA, Weinshilboum RM (2005) Human aromatase: gene resequencing and functional genomics. Cancer Res 65(23):11071–11082. doi:10.1158/0008-5472.CAN-05-1218
Tempfer CB, Hefler LA, Schneeberger C, Huber JC (2006) How valid is single nucleotide polymorphism (SNP) diagnosis for the individual risk assessment of breast cancer? Gynecol Endocrinol 22(3):155–159. doi:10.1080/09513590600629175
Mao JJ, Su HI, Feng R, Donelson ML, Aplenc R, Rebbeck TR, Stanczyk F, DeMichele A (2011) Association of functional polymorphisms in CYP19A1 with aromatase inhibitor associated arthralgia in breast cancer survivors. Breast Cancer Res 13(1):R8. doi:10.1186/bcr2813
Enjuanes A, Garcia-Giralt N, Supervia A, Nogues X, Ruiz-Gaspa S, Bustamante M, Mellibovsky L, Grinberg D, Balcells S, Diez-Perez A (2006) A new SNP in a negative regulatory region of the CYP19A1 gene is associated with lumbar spine BMD in postmenopausal women. Bone 38(5):738–743. doi:10.1016/j.bone.2005.10.010
Napoli N, Villareal DT, Mumm S, Halstead L, Sheikh S, Cagaanan M, Rini GB, Armamento-Villareal R (2005) Effect of CYP1A1 gene polymorphisms on estrogen metabolism and bone density. J Bone Miner Res 20(2):232–239. doi:10.1359/JBMR.041110
Zarrabeitia MT, Hernandez JL, Valero C, Zarrabeitia AL, Garcia-Unzueta M, Amado JA, Gonzalez-Macias J, Riancho JA (2004) A common polymorphism in the 5′-untranslated region of the aromatase gene influences bone mass and fracture risk. Eur J Endocrinol 150(5):699–704
Sowers MR, Wilson AL, Karvonen-Gutierrez CA, Kardia SR (2006) Sex steroid hormone pathway genes and health-related measures in women of 4 races/ethnicities: the Study of Women’s Health Across the Nation (SWAN). Am J Med 119(9 Suppl 1):S103–S110. doi:10.1016/j.amjmed.2006.07.012
Ingle JN, Schaid DJ, Goss PE, Liu M, Mushiroda T, Chapman JA, Kubo M, Jenkins GD, Batzler A, Shepherd L, Pater J, Wang L, Ellis MJ, Stearns V, Rohrer DC, Goetz MP, Pritchard KI, Flockhart DA, Nakamura Y, Weinshilboum RM (2010) Genome-wide associations and functional genomic studies of musculoskeletal adverse events in women receiving aromatase inhibitors. J Clin Oncol 28(31):4674–4682. doi:10.1200/JCO.2010.28.5064
Napoli N, Rastelli A, Ma C, Yarramaneni J, Vattikutti S, Moskowitz G, Giri T, Mueller C, Kulkarny V, Qualls C, Ellis M, Armamento-Villareal R (2013) Genetic polymorphism at Val80 (rs700518) of the CYP19A1 gene is associated with aromatase inhibitor associated bone loss in women with ER + breast cancer. Bone 55(2):309–314. doi:10.1016/j.bone.2013.04.021
Breast International Group 1–98 Collaborative G, Thurlimann B, Keshaviah A, Coates AS, Mouridsen H, Mauriac L, Forbes JF, Paridaens R, Castiglione-Gertsch M, Gelber RD, Rabaglio M, Smith I, Wardley A, Price KN, Goldhirsch A (2005) A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med 353(26):2747–2757. doi:10.1056/NEJMoa052258
Regan MM, Neven P, Giobbie-Hurder A, Goldhirsch A, Ejlertsen B, Mauriac L, Forbes JF, Smith I, Lang I, Wardley A, Rabaglio M, Price KN, Gelber RD, Coates AS, Thurlimann B (2011) Assessment of letrozole and tamoxifen alone and in sequence for postmenopausal women with steroid hormone receptor-positive breast cancer: the BIG 1–98 randomised clinical trial at 8.1 years median follow-up. Lancet Oncol 12(12):1101–1108. doi:10.1016/s1470-2045(11)70270-4
Regan MM, Leyland-Jones B, Bouzyk M, Pagani O, Tang W, Kammler R, Dell’orto P, Biasi MO, Thurlimann B, Lyng MB, Ditzel HJ, Neven P, Debled M, Maibach R, Price KN, Gelber RD, Coates AS, Goldhirsch A, Rae JM, Viale G, Breast International Group 1–98 Collaborative G (2012) CYP2D6 genotype and tamoxifen response in postmenopausal women with endocrine-responsive breast cancer: the Breast International Group 1–98 trial. J Natl Cancer Inst 104(6):441–451. doi:10.1093/jnci/djs125
Rae JM, Regan MM, Thibert JN, Gersch C, Thomas D, Leyland-Jones B, Viale G, Pusztai L, Hayes DF, Skaar T, Van Poznak C (2013) Concordance between CYP2D6 genotypes obtained from tumor-derived and germline DNA. J Natl Cancer Inst 105(17):1332–1334
McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM, Statistics Subcommittee of the NCIEWGoCD (2005) Reporting recommendations for tumor marker prognostic studies. J Clin Oncol 23(36):9067–9072. doi:10.1200/JCO.2004.01.0454
Chen C, Sakoda LC, Doherty JA, Loomis MM, Fish S, Ray RM, Lin MG, Fan W, Zhao LP, Gao DL, Stalsberg H, Feng Z, Thomas DB (2008) Genetic variation in CYP19A1 and risk of breast cancer and fibrocystic breast conditions among women in Shanghai, China. Cancer Epidemiol Biomarkers Prev 17(12):3457–3466. doi:10.1158/1055-9965.EPI-08-0517
Fasching PA, Loehberg CR, Strissel PL, Lux MP, Bani MR, Schrauder M, Geiler S, Ringleff K, Oeser S, Weihbrecht S, Schulz-Wendtland R, Hartmann A, Beckmann MW, Strick R (2008) Single nucleotide polymorphisms of the aromatase gene (CYP19A1), HER2/neu status, and prognosis in breast cancer patients. Breast Cancer Res Treat 112(1):89–98. doi:10.1007/s10549-007-9822-2
Garcia-Casado Z, Guerrero-Zotano A, Llombart-Cussac A, Calatrava A, Fernandez-Serra A, Ruiz-Simon A, Gavila J, Climent MA, Almenar S, Cervera-Deval J, Campos J, Albaladejo CV, Llombart-Bosch A, Guillem V, Lopez-Guerrero JA (2010) A polymorphism at the 3′-UTR region of the aromatase gene defines a subgroup of postmenopausal breast cancer patients with poor response to neoadjuvant letrozole. BMC Cancer 10:36. doi:10.1186/1471-2407-10-36
Lunardi G, Piccioli P, Bruzzi P, Notaro R, Lastraioli S, Serra M, Marroni P, Bighin C, Mansutti M, Puglisi F, Porpiglia M, Ponzone R, Bisagni G, Garrone O, Cavazzini G, Clavarezza M, Del Mastro L (2013) Plasma estrone sulfate concentrations and genetic variation at the CYP19A1 locus in postmenopausal women with early breast cancer treated with letrozole. Breast Cancer Res Treat 137(1):167–174. doi:10.1007/s10549-012-2306-z
Colomer R, Monzo M, Tusquets I, Rifa J, Baena JM, Barnadas A, Calvo L, Carabantes F, Crespo C, Munoz M, Llombart A, Plazaola A, Artells R, Gilabert M, Lloveras B, Alba E (2008) A single-nucleotide polymorphism in the aromatase gene is associated with the efficacy of the aromatase inhibitor letrozole in advanced breast carcinoma. Clin Cancer Res 14(3):811–816. doi:10.1158/1078-0432.CCR-07-1923
Ferraldeschi R, Arnedos M, Hadfield KD, A’Hern R, Drury S, Wardley A, Howell A, Evans DG, Roberts SA, Smith I, Newman WG, Dowsett M (2012) Polymorphisms of CYP19A1 and response to aromatase inhibitors in metastatic breast cancer patients. Breast Cancer Res Treat 133(3):1191–1198. doi:10.1007/s10549-012-2010-z
Liu L, Bai YX, Zhou JH, Sun XW, Sui H, Zhang WJ, Yuan HH, Xie R, Wei XL, Zhang TT, Huang P, Li YJ, Wang JX, Zhao S, Zhang QY (2013) A polymorphism at the 3′-UTR region of the aromatase gene is associated with the efficacy of the aromatase inhibitor, anastrozole, in metastatic breast carcinoma. Int J Mol Sci 14(9):18973–18988. doi:10.3390/ijms140918973
Clendenen T, Zeleniuch-Jacquotte A, Wirgin I, Koenig KL, Afanasyeva Y, Lundin E, Arslan AA, Axelsson T, Forsti A, Hallmans G, Hemminki K, Lenner P, Roy N, Shore RE, Chen Y (2013) Genetic variants in hormone-related genes and risk of breast cancer. PLoS One 8(7):e69367. doi:10.1371/journal.pone.0069367
Haiman CA, Stram DO, Pike MC, Kolonel LN, Burtt NP, Altshuler D, Hirschhorn J, Henderson BE (2003) A comprehensive haplotype analysis of CYP19 and breast cancer risk: the Multiethnic Cohort. Hum Mol Genet 12(20):2679–2692. doi:10.1093/hmg/ddg294
Lee KM, Abel J, Ko Y, Harth V, Park WY, Seo JS, Yoo KY, Choi JY, Shin A, Ahn SH, Noh DY, Hirvonen A, Kang D (2003) Genetic polymorphisms of cytochrome P450 19 and 1B1, alcohol use, and breast cancer risk in Korean women. Br J Cancer 88(5):675–678. doi:10.1038/sj.bjc.6600761
Miyoshi Y, Iwao K, Ikeda N, Egawa C, Noguchi S (2000) Breast cancer risk associated with polymorphism in CYP19 in Japanese women. Int J Cancer (Journal international du cancer) 89(4):325–328
Probst-Hensch NM, Ingles SA, Diep AT, Haile RW, Stanczyk FZ, Kolonel LN, Henderson BE (1999) Aromatase and breast cancer susceptibility. Endocr Relat Cancer 6(2):165–173
Watanabe J, Harada N, Suemasu K, Higashi Y, Gotoh O, Kawajiri K (1997) Arginine-cysteine polymorphism at codon 264 of the human CYP19 gene does not affect aromatase activity. Pharmacogenetics 7(5):419–424
Cai Q, Kataoka N, Li C, Wen W, Smith JR, Gao YT, Shu XO, Zheng W (2008) Haplotype analyses of CYP19A1 gene variants and breast cancer risk: results from the Shanghai Breast Cancer Study. Cancer Epidemiol Biomarkers Prev 17(1):27–32. doi:10.1158/1055-9965.EPI-07-0688
Mao JJ, Stricker C, Bruner D, Xie S, Bowman MA, Farrar JT, Greene BT, DeMichele A (2009) Patterns and risk factors associated with aromatase inhibitor-related arthralgia among breast cancer survivors. Cancer 115(16):3631–3639. doi:10.1002/cncr.24419
Fontein DB, Houtsma D, Nortier JW, Baak-Pablo RF, Kranenbarg EM, van der Straaten TR, Putter H, Seynaeve C, Gelderblom H, van de Velde CJ, Guchelaar HJ (2014) Germline variants in the CYP19A1 gene are related to specific adverse events in aromatase inhibitor users: a substudy of Dutch patients in the TEAM trial. Breast Cancer Res Treat 144(3):599–606. doi:10.1007/s10549-014-2873-2
Acknowledgments
The BIG 1–98 trial is registered at ClinicalTrials.gov (Identifier NCT00004205). We are indebted to the women, physicians, nurses, and data managers who participated in this clinical trial; to the many pathologists who submitted tumor blocks; to the BIG 1–98 Steering Committee; to Novartis for funding of the clinical trial and of the collection of tumor blocks; to the IBCSG for the design of the trial, coordination, data management, medical review, and statistical support; to the IBCSG Central Pathology Office for collection and processing of tumor blocks; to the BIG-198 Collaborative Group (members who submitted tumor blocks are listed in Supplementary Appendix, available online); to Susan G. Komen for the Cure Promise Grant. The translational research, including DNA extraction and genotyping, was funded by Susan G. Komen for the Cure Promise Grant (KG080081 to GV, OP, MMR) and by The Breast Cancer Research Foundation (BCRF) (N003173 to JMR), the National Institutes of Health (1RO1GM099143 to JMR). The Breast International Group (BIG) 1–98 trial was funded by Novartis and coordinated by the International Breast Cancer Study Group (IBCSG). Other support for the IBCSG: United States National Cancer Institute (CA75362 to MMR).
Compliance with ethical standards
Experiments comply with the current laws of the country in which they were performed. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The BIG 1–98 trial was funded by Novartis, who contracted with the IBCSG for provision of services related to the conduct and management of the trial, and provided partial support for collection and review of tumor blocks. Drs. Goldhirsch and Coates are responsible for the scientific management of the IBCSG. Dr. Viale is responsible for management of the IBCSG Central Pathology Office. Dr. Thürlimann owns stock in Novartis. Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The remaining authors have no conflicts to declare.
Author information
Authors and Affiliations
Corresponding author
Additional information
On behalf of the BIG 1–98 Collaborative Group.
Members of the BIG 1–98 Collaborative Group who submitted tumor blocks are listed in Supplementary Appendix.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Leyland-Jones, B., Gray, K.P., Abramovitz, M. et al. CYP19A1 polymorphisms and clinical outcomes in postmenopausal women with hormone receptor-positive breast cancer in the BIG 1–98 trial. Breast Cancer Res Treat 151, 373–384 (2015). https://doi.org/10.1007/s10549-015-3378-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-015-3378-3