Abstract
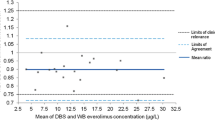
The anti-estrogenic effect of tamoxifen is suggested to be mainly attributable to its metabolite (Z)-endoxifen, and a minimum therapeutic threshold for (Z)-endoxifen in serum has been proposed. The objective of this research was to establish the relationship between dried blood spot (DBS) and serum concentrations of tamoxifen and (Z)-endoxifen to allow the use of DBS sampling, a simple and patient-friendly alternative to venous sampling, in clinical practice. Paired DBS and serum samples were obtained from 50 patients using tamoxifen and analyzed using HPLC-MS/MS. Serum concentrations were calculated from DBS concentrations using the formula calculated serum concentration = DBS concentration/([1-haematocrit (Hct)] + blood cell-to-serum ratio × Hct). The blood cell-to-serum ratio was determined ex vivo by incubating a batch of whole blood spiked with both analytes. The average Hct for female adults was imputed as a fixed value. Calculated and analyzed serum concentrations were compared using weighted Deming regression. Weighted Deming regression analysis comparing 44 matching pairs of DBS and serum samples showed a proportional bias for both analytes. Serum concentrations were calculated using [Tamoxifen] serum, calculated = [Tamoxifen] DBS /0.779 and [(Z)-Endoxifen] serum, calculated = [(Z)-Endoxifen] DBS /0.663. Calculated serum concentrations were within 20 % of analyzed serum concentrations in 84 and 100 % of patient samples for tamoxifen and (Z)-endoxifen, respectively. In conclusion, DBS concentrations of tamoxifen and (Z)-endoxifen were equal to serum concentrations after correction for Hct and blood cell-to-serum ratio. DBS sampling can be used in clinical practice.



Similar content being viewed by others
References
Teunissen SF, Rosing H, Seoane MD et al (2011) Investigational study of tamoxifen phase I metabolites using chromatographic and spectroscopic analytical techniques. J Pharm Biomed Anal 55:518–526
Murdter TE, Schroth W, Bacchus-Gerybadze L et al (2011) Activity levels of tamoxifen metabolites at the estrogen receptor and the impact of genetic polymorphisms of phase I and II enzymes on their concentration levels in plasma. Clin Pharmacol Ther 89:708–717
IARC Working Group (2006) Tamoxifen Monograph. IARC 131–153
Lim YC, Desta Z, Flockhart DA, Skaar TC (2005) Endoxifen (4-hydroxy-N-desmethyl-tamoxifen) has anti-estrogenic effects in breast cancer cells with potency similar to 4-hydroxy-tamoxifen. Cancer Chemother Pharmacol 55:471–478
Johnson MD, Zuo H, Lee KH et al (2004) Pharmacological characterization of 4-hydroxy-N-desmethyl tamoxifen, a novel active metabolite of tamoxifen. Breast Cancer Res Treat 85:151–159
Lu WJ, Desta Z, Flockhart DA (2012) Tamoxifen metabolites as active inhibitors of aromatase in the treatment of breast cancer. Breast Cancer Res Treat 131:473–481. doi:10.1007/s10549-011-1428-z
Wu X, Hawse JR, Subramaniam M et al (2009) The tamoxifen metabolite, endoxifen, is a potent antiestrogen that targets estrogen receptor alpha for degradation in breast cancer cells. Cancer Res 69:1722–1727. doi:10.1158/0008-5472.CAN-08-3933
Hawse JR, Subramaniam M, Cicek M et al (2013) Endoxifen’s molecular mechanisms of action are concentration dependent and different than that of other anti-estrogens. PLoS ONE 8:e54613. doi:10.1371/journal.pone.0054613
Gong IY, Teft W, Ly J et al (2013) Determination of clinically therapeutic endoxifen concentrations based on efficacy from human MCF7 breast cancer xenografts. Breast Cancer Res Treat 450:61–69. doi:10.1007/s10549-013-2530-1
Madlensky L, Natarajan L, Tchu S et al (2011) Tamoxifen metabolite concentrations, CYP2D6 genotype, and breast cancer outcomes. Clin Pharmacol Ther 89:718–725
Edelbroek PM, van der Heijden J, Stolk LML (2009) Dried blood spot methods in therapeutic drug monitoring: methods, assays, and pitfalls. Ther Drug Monit 31:327–336. doi:10.1097/FTD.0b013e31819e91ce
Li W, Tse FLS (2010) Dried blood spot sampling in combination with LC-MS/MS for quantitative analysis of small molecules. Biomed Chromatogr 24:49–65. doi:10.1002/bmc.1367
Clinical and Laboratory Standards Institute (2002) Method comparison and bias estimation using patient samples; Approved guideline—EP-09-A2. Wayne, USA
Jager NGL, Rosing H, Schellens JHM et al (2014) Tamoxifen dose and serum concentrations of tamoxifen and six of its metabolites in routine clinical outpatient care. Breast Cancer Res Treat 143:477–483. doi:10.1007/s10549-013-2826-1
Jager NGL, Rosing H, Schellens JHM, Beijnen JH, Linn SC (2014) Determination of tamoxifen and endoxifen in dried blood spots using LC-MS/MS and the effect of coated DBS cards on recovery and matrix effect. Bioanalysis. Accepted manuscript
Teunissen SF, Jager NGL, Rosing H et al (2011) Development and validation of a quantitative assay for the determination of tamoxifen and its five main phase I metabolites in human serum using liquid chromatography coupled with tandem mass spectrometry. J Chromatogr B 879:1677–1685
Jager NGL, Rosing H, Linn SC et al (2012) Importance of highly selective LC-MS/MS analysis for the accurate quantification of tamoxifen and its metabolites: focus on endoxifen and 4-hydroxytamoxifen. Breast Cancer Res Treat 133:793–798. doi:10.1007/s10549-012-2000-1
Food and Drug Administration (2001) Guidance for Industry: Bioanalytical Method Validation. U.S. Department of Health and Human Services 4–10
European Medicines Agency (2011) Guideline on Bioanalytical Method Validation. European Medicines Agency 4–10
Rowland M, Emmons GT (2010) Use of dried blood spots in drug development: pharmacokinetic considerations. AAPS J 12:290–293. doi:10.1208/s12248-010-9188-y
Wickremsinhe E, Abdul B, Huang N et al (2011) Dried blood spot sampling: coupling bioanalytical feasibility, blood-plasma partitioning and transferability to in vivo preclinical studies. Bioanalysis 3:1635–1646
Dhungana S, Meng M, Allen MS (2012) Boost drug discovery efficiency—switching from plasma to dried blood spots. White Pap Tandem Labs 1–8
Wickremsinhe E, Huang N, Abdul B (2013) Preclinical bridging studies: understanding dried blood spot and plasma exposure profiles. Bioanalysis 5:159–170
Le T, Bhushan V (2013) First aid for the USMLE Step 1, 23rd ed. xxii
Van der Elst KCM, Span LFR, van Hateren K et al (2013) Dried blood spot analysis suitable for therapeutic drug monitoring of voriconazole, fluconazole, and posaconazole. Antimicrob Agents Chemother 57:4999–5004. doi:10.1128/AAC.00707-13
Berm EJJ, Brummel-Mulder E, Paardekooper J et al (2014) Determination of venlafaxine and O-desmethylvenlafaxine in dried blood spots for TDM purposes, using LC-MS/MS. Anal Bioanal Chem 406:2349–2353. doi:10.1007/s00216-014-7619-9
Kong ST, Lim S-H, Chan E, Ho PC (2013) Estimation and comparison of carbamazepine population pharmacokinetics using dried blood spot and plasma concentrations from people with epilepsy: the clinical implication. J Clin Pharmacol 54:225–233. doi:10.1002/jcph.170
Eyles D, Anderson C, Ko P et al (2009) A sensitive LC/MS/MS assay of 25OH vitamin D3 and 25OH vitamin D2 in dried blood spots. Clin Chim Acta 403:145–151. doi:10.1016/j.cca.2009.02.005
Della Bona ML, Malvagia S, Villanelli F et al (2013) A rapid liquid chromatography tandem mass spectrometry-based method for measuring propranolol on dried blood spots. J Pharm Biomed Anal 78–79:34–38. doi:10.1016/j.jpba.2013.01.034
Mercolini L, Mandrioli R, Gerra G, Raggi MA (2010) Analysis of cocaine and two metabolites in dried blood spots by liquid chromatography with fluorescence detection: a novel test for cocaine and alcohol intake. J Chromatogr A 1217:7242–7248. doi:10.1016/j.chroma.2010.09.037
Mercolini L, Mandrioli R, Sorella V et al (2013) Dried blood spots: liquid chromatography-mass spectrometry analysis of Δ9-tetrahydrocannabinol and its main metabolites. J Chromatogr A 1271:33–40. doi:10.1016/j.chroma.2012.11.030
Kralj E, Trontelj J, Pajič T, Kristl A (2012) Simultaneous measurement of imatinib, nilotinib and dasatinib in dried blood spot by ultra high performance liquid chromatography tandem mass spectrometry. J Chromatogr B 903:150–156. doi:10.1016/j.jchromb.2012.07.011
Arpini J, Antunes MV, Pacheco LS et al (2013) Clinical evaluation of a dried blood spot method for determination of mycophenolic acid in renal transplant patients. Clin Biochem 46:1905–1908. doi:10.1016/j.clinbiochem.2013.10.011
Kromdijk W, Mulder JW, Rosing H et al (2012) Use of dried blood spots for the determination of plasma concentrations of nevirapine and efavirenz. J Antimicrob Chemother 67:1211–1216. doi:10.1093/jac/dks011
Taylor RR, Hoffman KL, Schniedewind B et al (2013) Comparison of the quantification of acetaminophen in plasma, cerebrospinal fluid and dried blood spots using high-performance liquid chromatography-tandem mass spectrometry. J Pharm Biomed Anal 83:1–9. doi:10.1016/j.jpba.2013.04.007
Davies C, Godwin J, Gray R et al (2011) Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet 378:771–784. doi:10.1016/S0140-6736(11)60993-8
Acknowledgments
We would like to acknowledge the assistance of the nurse practitioners of the Breast Cancer Clinic of the Netherlands Cancer Institute in patient recruitment. We would like to thank the staff of the Trial Laboratory of the Netherlands Cancer Institute for obtaining all patient samples.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jager, N.G.L., Rosing, H., Schellens, J.H.M. et al. Use of dried blood spots for the determination of serum concentrations of tamoxifen and endoxifen. Breast Cancer Res Treat 146, 137–144 (2014). https://doi.org/10.1007/s10549-014-2999-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-014-2999-2