Abstract
Purpose
Tamoxifen is an effective drug for the treatment and prevention of breast cancer. It is extensively metabolized by the human cytochrome P450 enzyme system into several metabolites. Of these, 4-hydroxy-tamoxifen (4-OH-Tam) is an active metabolite, which has greater anti-estrogenic potency than the parent drug, tamoxifen. We reported recently that 4-hydroxy-N-desmethyl-tamoxifen (endoxifen) could also be active. The progesterone receptor (PR) messenger ribonucleic acid (mRNA) expression is commonly studied as a marker of estrogenic effect in breast cancer cells and PR levels in breast cancer patients are correlated with tamoxifen response. We, therefore, determined the effect of endoxifen and 4-OH-Tam on 17β-estradiol (E2)-induced PR mRNA expression in an estrogen receptor-positive human breast cancer cell line.
Methods
MCF-7 cells were treated with drugs for 24 h. The total ribonucleic acid (RNA) was harvested and transcribed into complementary deoxyribonucleic acids (cDNAs). The PR mRNA level was measured by using real-time reverse transcription polymerase chain reaction (RT-PCR). The PR expression data were normalized using a glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene expression. We measured the metabolite concentrations in the cultured media by high performance liquid chromatography (HPLC) to determine whether there was conversion of one metabolite to the other.
Results
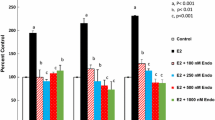
Consistent with previous reports, the dose–response of the E2 effect on the PR expression indicated an ED50 value of approximately 60 pM and the maximum induction of PR mRNA was nearly ten-fold. When 10−10 M E2 was used, induction of the PR expression was observed in 2 h and reached its maximum at 24 h. In this assay, neither endoxifen nor 4-OH-Tam alone produced any change in the PR mRNA expression. However, both endoxifen and 4-OH-Tam decreased the E2-induced PR expression with similar potency. There was very little interconversion between the two metabolites during the culture.
Conclusions
Since endoxifen is present at greater concentrations than 4-OH-Tam in human plasma of breast cancer patients receiving chronic tamoxifen, these results provide further evidence that endoxifen is as important as, or more important than, 4-OH-Tam to the anti-estrogenic action of tamoxifen.





Similar content being viewed by others
References
Allan GF, Hutchins A, Liu X, Clancy J (2001) Induction of the progesterone receptor gene in estrogen target cells monitored by branched DNA signal amplification. Steroids 66:663–671
Bardou VJ, Arpino G, Elledge RM, Osborne CK, Clark GM (2003) Progesterone receptor status significantly improves outcome prediction over estrogen receptor status alone for adjuvant endocrine therapy in two large breast cancer databases. J Clin Oncol 21:1973–1979
Bernstein L, Deapen D, Cerhan JR, Schwartz SM, Liff J, McGann-Maloney E, Perlman JA, Ford L (1999) Tamoxifen therapy for breast cancer and endometrial cancer risk. J Natl Cancer Inst 91:1654–1662
Bethea CL, Widmann AA (1998) Differential expression of progestin receptor isoforms in the hypothalamus, pituitary, and endometrium of rhesus macaques. Endocrinology 139:677–687
Bonomi P, Gale M, Von Roenn J, Anderson K, Johnson P, Wolter J, Economou S (1988) Quantitative estrogen and progesterone receptor levels related to progression-free interval in advanced breast cancer patients treated with megestrol acetate or tamoxifen. Semin Oncol 15:26–33
Clayton SJ, May FE, Westley BR (1997) Insulin-like growth factors control the regulation of oestrogen and progesterone receptor expression by oestrogens. Mol Cell Endocrinol 128:57–68
Coezy E, Borgna JL, Rochefort H (1982) Tamoxifen and metabolites in MCF7 cells: correlation between binding to estrogen receptor and inhibition of cell growth. Cancer Res 42:317–323
Crewe HK, Notley LM, Wunsch RM, Lennard MS, Gillam EM (2002) Metabolism of tamoxifen by recombinant human cytochrome P450 enzymes: formation of the 4-hydroxy, 4′-hydroxy and N-desmethyl metabolites and isomerization of trans-4-hydroxytamoxifen. Drug Metab Dispos 30:869–874
Cuzick J, Powles T, Veronesi U, Forbes J, Edwards R, Ashley S, Boyle P (2003) Overview of the main outcomes in breast-cancer prevention trials. Lancet 361:296–300
Decensi A, Robertson C, Viale G, Pigatto F, Johansson H, Kisanga ER, Veronesi P, Torrisi R, Cazzaniga M, Mora S, Sandri MT, Pelosi G, Luini A, Goldhirsch A, Lien EA, Veronesi U (2003) A randomized trial of low-dose tamoxifen on breast cancer proliferation and blood estrogenic biomarkers. J Natl Cancer Inst 95:779–790
Desta Z, Ward BA, Soukhova NV, Flockhart DA (2004) Comprehensive evaluation of tamoxifen sequential biotransformation by the human cytochrome P450 system in vitro: prominent roles for CYP3A and CYP2D6. J Pharmacol Exp Ther 310(3):1062–1075
Detre S, Riddler S, Salter J, A’Hern R, Dowsett M, Johnston SR (2003) Comparison of the selective estrogen receptor modulator arzoxifene (LY353381) with tamoxifen on tumor growth and biomarker expression in an MCF-7 human breast cancer xenograft model. Cancer Res 63:6516–6522
Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, Vogel V, Robidoux A, Dimitrov N, Atkins J, Daly M, Wieand S, Tan-Chiu E, Ford L, Wolmark N (1998) Tamoxifen for prevention of breast cancer: report of the national surgical adjuvant breast and bowel project P-1 study. J Natl Cancer Inst 90:1371–1388
Fried KM, Wainer IW (1994) Direct determination of tamoxifen and its four major metabolites in plasma using coupled column high-performance liquid chromatography. J Chromatogr B Biomed Appl 655:261–268
Fujimoto J, Ichigo S, Hirose R, Sakaguchi H, Tamaya T (1997) Clinical implication of expression of progesterone receptor form A and B mRNAs in secondary spreading of gynecologic cancers. J Steroid Biochem Mol Biol 62:449–454
Gail MH, Costantino JP, Bryant J, Croyle R, Freedman L, Helzlsouer K, Vogel V (1999) Weighing the risks and benefits of tamoxifen treatment for preventing breast cancer. J Natl Cancer Inst 91:1829–1846
Giangrande PH, Kimbrel EA, Edwards DP, McDonnell DP (2000) The opposing transcriptional activities of the two isoforms of the human progesterone receptor are due to differential cofactor binding. Mol Cell Biol 20:3102–3115
Howell A, Howell SJ, Evans DG (2003) New approaches to the endocrine prevention and treatment of breast cancer. Cancer Chemother Pharmacol 52(Suppl 1):S39–S44
Ingle JN, Suman VJ, Johnson PA, Krook JE, Mailliard JA, Wheeler RH, Loprinzi CL, Perez EA, Jordan VC, Dowsett M (1999) Evaluation of tamoxifen plus letrozole with assessment of pharmacokinetic interaction in postmenopausal women with metastatic breast cancer. Clin Cancer Res 5:1642–1649
Johnson MD, Zuo H, Lee KH, Trebley JP, Rae JM, Weatherman RV, Desta Z, Morocho A, Flockhart DA, Skaar TC (2004) Pharmacological characterization of 4-hydroxy-N-desmethyl tamoxifen, a novel active metabolite of tamoxifen. Breast Cancer Res Treat 207:1–9
Kastner P, Krust A, Turcotte B, Stropp U, Tora L, Gronemeyer H, Chambon P (1990) Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. EMBO J 9:1603–1614
Katzenellenbogen BS, Norman MJ, Eckert RL, Peltz SW, Mangel WF (1984) Bioactivities, estrogen receptor interactions, and plasminogen activator-inducing activities of tamoxifen and hydroxy-tamoxifen isomers in MCF-7 human breast cancer cells. Cancer Res 44:112–119
Katzenellenbogen JA, Carlson KE, Katzenellenbogen BS (1985) Facile geometric isomerization of phenolic non-steroidal estrogens and antiestrogens: limitations to the interpretation of experiments characterizing the activity of individual isomers. J Steroid Biochem 22:589–596
Kikuta C, Schmid R (1989) Specific high-performance liquid chromatographic analysis of tamoxifen and its major metabolites by “on-line” extraction and post-column photochemical reaction. J Pharm Biomed Anal 7:329–337
Kivisto KT, Villikka K, Nyman L, Anttila M, Neuvonen PJ (1998) Tamoxifen and toremifene concentrations in plasma are greatly decreased by rifampin. Clin Pharmacol Ther 64:648–654
Lamy PJ, Pujol P, Thezenas S, Kramar A, Rouanet P, Guilleux F, Grenier J (2002) Progesterone receptor quantification as a strong prognostic determinant in postmenopausal breast cancer women under tamoxifen therapy. Breast Cancer Res Treat 76:65–71
Lee KH, Ward BA, Desta Z, Flockhart DA, Jones DR (2003) Quantification of tamoxifen and three metabolites in plasma by high-performance liquid chromatography with fluorescence detection: application to a clinical trial. J Chromatogr B Analyt Technol Biomed Life Sci 791:245–253
Lien EA, Solheim E, Kvinnsland S, Ueland PM (1988) Identification of 4-hydroxy-N-desmethyltamoxifen as a metabolite of tamoxifen in human bile. Cancer Res 48:2304–2308
Lien EA, Solheim E, Lea OA, Lundgren S, Kvinnsland S, Ueland PM (1989) Distribution of 4-hydroxy-N-desmethyltamoxifen and other tamoxifen metabolites in human biological fluids during tamoxifen treatment. Cancer Res 49:2175–2183
Lien EA, Solheim E, Ueland PM (1991) Distribution of tamoxifen and its metabolites in rat and human tissues during steady-state treatment. Cancer Res 51:4837–4844
May FE, Johnson MD, Wiseman LR, Wakeling AE, Kastner P, Westley BR (1989) Regulation of progesterone receptor mRNA by oestradiol and antioestrogens in breast cancer cell lines. J Steroid Biochem 33:1035–1041
Meier CR, Jick H (1998) Tamoxifen and risk of idiopathic venous thromboembolism. Br J Clin Pharmacol 45:608–612
Murphy CS, Langan-Fahey SM, McCague R, Jordan VC (1990) Structure–function relationships of hydroxylated metabolites of tamoxifen that control the proliferation of estrogen-responsive T47D breast cancer cells in vitro. Mol Pharmacol 38:737–743
Murphy CS, Parker CJ, McCague R, Jordan VC (1991) Structure–activity relationships of nonisomerizable derivatives of tamoxifen: importance of hydroxyl group and side chain positioning for biological activity. Mol Pharmacol 39:421–428
Osborne CK (1998) Tamoxifen in the treatment of breast cancer. N Engl J Med 339:1609–1618
Ravdin PM, Green S, Dorr TM, McGuire WL, Fabian C, Pugh RP, Carter RD, Rivkin SE, Borst JR, Belt RJ, Metch B, Osborne CK (1992) Prognostic significance of progesterone receptor levels in estrogen receptor-positive patients with metastatic breast cancer treated with tamoxifen: results of a prospective Southwest oncology group study. J Clin Oncol 10:1284–1291
Robertson DW, Katzenellenbogen JA, Long DJ, Rorke EA, Katzenellenbogen BS (1982) Tamoxifen antiestrogens. A comparison of the activity, pharmacokinetics, and metabolic activation of the cis and trans isomers of tamoxifen. J Steroid Biochem 16:1–13
Sartorius CA, Melville MY, Hovland AR, Tung L, Takimoto GS, Horwitz KB (1994) A third transactivation function (AF3) of human progesterone receptors located in the unique N-terminal segment of the B-isoform. Mol Endocrinol 8:1347–1360
Shao W, Brown M (2004) Advances in estrogen receptor biology: prospects for improvements in targeted breast cancer therapy. Breast Cancer Res 6:39–52
Stearns V, Johnson MD, Rae JM, Morocho A, Novielli A, Bhargava P, Hayes DF, Desta Z, Flockhart DA (2003) Active tamoxifen metabolite plasma concentrations after coadministration of tamoxifen and the selective serotonin reuptake inhibitor paroxetine. J Natl Cancer Inst 95:1758–1764
Wei LL, Hawkins P, Baker C, Norris B, Sheridan PL, Quinn PG (1996) An amino-terminal truncated progesterone receptor isoform, PRc, enhances progestin-induced transcriptional activity. Mol Endocrinol 10:1379–1387
Wei LL, Miner R (1994) Evidence for the existence of a third progesterone receptor protein in human breast cancer cell line T47D. Cancer Res 54:340–343
Acknowledgments
This work was supported in part by a Pharmacogenetic Network Grant from the NIGMS, Bethesda, Maryland, USA (U-01-GM61373), by a Clinical Pharmacology Training Grant to the Division of Clinical Pharmacology at Indiana University School of Medicine, Indiana, USA (T-32-GM04825), and by a PhRMA Foundation Grant (Indiana Center for Excellence in Clinical Pharmacology, Indiana, USA).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lim, Y.C., Desta, Z., Flockhart, D.A. et al. Endoxifen (4-hydroxy-N-desmethyl-tamoxifen) has anti-estrogenic effects in breast cancer cells with potency similar to 4-hydroxy-tamoxifen. Cancer Chemother Pharmacol 55, 471–478 (2005). https://doi.org/10.1007/s00280-004-0926-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-004-0926-7