Abstract
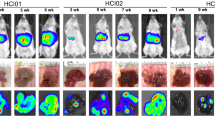
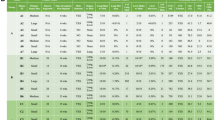
Breast carcinoma is comprised of heterogeneous groups of cells with different metastatic potential. To develop effective therapeutic strategies targeting metastatic disease, it is crucial to understand the characteristics of breast cancer cells that enable metastasis to distant organs. 4THM breast carcinoma cells are the cells of 4T1 primary tumors that metastasized to the heart. Cells of 4THM tumors which metastasized to liver (4TLM) were previously isolated. Recently macroscopic brain metastasis in 4THM injected animals, were isolated to obtain a brain metastatic cell line (4TBM). Using an orthotopic mouse model differential characteristic of cells metastasized to heart (4THM), liver (4TLM), and brain (4TBM) were compared for ability to metastasize and expression of stem cell markers. We found that 4TLM cells produced significantly more lung and liver metastasis compared to 4TBM and 4THM cells. In vitro, proliferation as well as migration rate of 4TLM cells was also significantly higher than the other cell lines. Remarkably primary tumors formed by 4TLM cells expressed significant amounts of CD34, a marker for mesenchymal malignancies. Markers of epithelial–mesenchymal transition were expressed in all metastatic cells, but the degree of expression differed. Majorities of 4TLM, 4THM, and 4TBM cells were CD44+ CD24− whereas, 12 % of 4TLM cells also expressed membranous CD24. Conditioned mediums of non-metastatic 67NR breast tumors and cancer-associated fibroblasts inhibited growth of highly metastatic 4TLM cells. Malignant cells metastasized to brain were distinguished by membranous E-cadherin expression that was markedly higher in 4TBM cells grown as spheroids suggesting E-cadherin is required for brain metastasis. Differential features of heart, brain, and liver metastatic cells in a syngenic model was shown in this study for the first time. These findings not only provide a model to explore new treatment modalities, but also demonstrate differential features of cancer cells that originally homed to a certain organ, such as liver or brain.







Similar content being viewed by others
Abbreviations
- CSC:
-
Cancer stem cells
- EMT:
-
Epithelial–mesenchymal transition
- VEGF:
-
Vascular endothelial growth factor
- SDF 1:
-
Stromal cell-derived factor-1
- CM:
-
Conditioned mediums
- CAF:
-
Cancer-associated fibroblasts
- α-SMA:
-
α-Smooth muscle actin
References
Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM (2010) Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 127:2893–2917
Sakorafas GH, Tsiotou AG (2000) Ductal carcinoma in situ (DCIS) of the breast: evolving perspectives. Cancer Treat Rev 26:103–125
Valastyan S, Weinberg RA (2011) Tumor metastasis: molecular insights and evolving paradigms. Cell 147:275–292
Hess KR, Varadhachary GR, Taylor SH, Wei W, Raber MN, Lenzi R, Abbruzzese JL (2006) Metastatic patterns in adenocarcinoma. Cancer 106:1624–1633
Liu R, Wang X, Chen GY, Dalerba P, Gurney A, Hoey T, Sherlock G, Lewicki J, Shedden K, Clarke MF (2007) The prognostic role of a gene signature from tumorigenic breast-cancer cells. N Engl J Med 356:217–226
Charafe-Jauffret E, Ginestier C, Iovino F, Wicinski J, Cervera N, Finetti P, Hur MH, Diebel ME, Monville F, Dutcher J, Brown M, Viens P, Xerri L, Bertucci F, Stassi G, Dontu G, Birnbaum D, Wicha MS (2009) Breast cancer cell lines contain functional cancer stem cells with metastatic capacity and a distinct molecular signature. Cancer Res 69:1302–1313
Abraham BK, Fritz P, McClellan M, Hauptvogel P, Athelogou M, Brauch H (2005) Prevalence of CD44 +/CD24-/low cells in breast cancer may not be associated with clinical outcome but may favor distant metastasis. Clin Cancer Res 11:1154–1159
Nathoo N, Chahlavi A, Barnett GH, Toms SA (2005) Pathobiology of brain metastases. J Clin Pathol 58:237–242
Patel RR, Mehta MP (2007) Targeted therapy for brain metastases: improving the therapeutic ratio. Clin Cancer Res 13:1675–1683
Gondi V, Mehta MP (2010) Novel insights into the management of brain metastases. Curr Opin Neurol 23:556–562
Tampellini M, Berruti A, Gerbino A, Buniva T, Torta M, Gorzegno G, Faggiuolo R, Cannone R, Farris A, Destefanis M, Moro G, Deltetto F, Dogliotti L (1997) Relationship between CA 15–3 serum levels and disease extent in predicting overall survival of breast cancer patients with newly diagnosed metastatic disease. Br J Cancer 75:698–702
Steeg PS, Theodorescu D (2008) Metastasis: a therapeutic target for cancer. Nat Clin Pract Oncol 5:206–219
Erin N, Akdas BG, Harms JF, Clawson GA (2008) Vagotomy enhances experimental metastases of 4THMpc breast cancer cells and alters substance P level. Regul Pept 151:35–42
Erin N, Boyer PJ, Bonneau RH, Clawson GA, Welch DR (2004) Capsaicin-mediated denervation of sensory neurons promotes mammary tumor metastasis to lung and heart. Anticancer Res 24:1003–1009
Erin N, Zhao W, Bylander J, Chase G, Clawson G (2006) Capsaicin-induced inactivation of sensory neurons promotes a more aggressive gene expression phenotype in breast cancer cells. Breast Cancer Res Treat 99:351–364
Erin N, Wang N, Xin P, Bui V, Weisz J, Barkan GA, Zhao W, Shearer D, Clawson GA (2009) Altered gene expression in breast cancer liver metastases. Int J Cancer 124:1503–1516
Aslakson CJ, Miller FR (1992) Selective events in the metastatic process defined by analysis of the sequential dissemination of subpopulations of a mouse mammary tumor. Cancer Res 52:1399–1405
Erin N, Duymus O, Ozturk S, Demir N (2012) Activation of vagus nerve by semapimod alters substance P levels and decreases breast cancer metastasis. Regul Pept 179:101–108
Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF (2003) Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A 100:3983–3988
Brown LF, Berse B, Van de Water L, Papadopoulos-Sergiou A, Perruzzi CA, Manseau EJ, Dvorak HF, Senger DR (1992) Expression and distribution of osteopontin in human tissues: widespread association with luminal epithelial surfaces. Mol Biol Cell 3:1169–1180
Zoller M (2011) CD44: can a cancer-initiating cell profit from an abundantly expressed molecule? Nat Rev Cancer 11:254–267
Sun H, Jia J, Wang X, Ma B, Di L, Song G, Ren J (2013) CD44(+)/CD24 (-) breast cancer cells isolated from MCF-7 cultures exhibit enhanced angiogenic properties. Clin Transl Oncol 15:46–54
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell LL, Polyak K, Brisken C, Yang J, Weinberg RA (2008) The epithelial–mesenchymal transition generates cells with properties of stem cells. Cell 133:704–715
Li W, Liu F, Lei T, Xu X, Liu B, Cui L, Wei J, Guo X, Lang R, Fan Y, Gu F, Tang P, Zhang X, Fu L (2010) The clinicopathological significance of CD44 +/CD24-/low and CD24 + tumor cells in invasive micropapillary carcinoma of the breast. Pathol Res Pract 206:828–834
Baumann P, Cremers N, Kroese F, Orend G, Chiquet-Ehrismann R, Uede T, Yagita H, Sleeman JP (2005) CD24 expression causes the acquisition of multiple cellular properties associated with tumor growth and metastasis. Cancer Res 65:10783–10793
Huber MA, Kraut N, Beug H (2005) Molecular requirements for epithelial–mesenchymal transition during tumor progression. Curr Opin Cell Biol 17:548–558
Nieman MT, Prudoff RS, Johnson KR, Wheelock MJ (1999) N-cadherin promotes motility in human breast cancer cells regardless of their E-cadherin expression. J Cell Biol 147:631–644
Perotti A, Sessa C, Mancuso A, Noberasco C, Cresta S, Locatelli A, Carcangiu ML, Passera K, Braghetti A, Scaramuzza D, Zanaboni F, Fasolo A, Capri G, Miani M, Peters WP, Gianni L (2009) Clinical and pharmacological phase I evaluation of Exherin (ADH-1), a selective anti-N-cadherin peptide in patients with N-cadherin-expressing solid tumours. Ann Oncol 20:741–745
Liu S, Miao Y, Fan C, Liu Y, Yu J, Zhang Y, Dai S, Wang E (2012) Clinicopathologic correlations of liver kinase B1, E-cadherin, and N-cadherin expression in non-small cell lung cancer. Appl Immunohistochem Mol Morphol
Gilles C, Polette M, Mestdagt M, Nawrocki-Raby B, Ruggeri P, Birembaut P, Foidart JM (2003) Transactivation of vimentin by beta-catenin in human breast cancer cells. Cancer Res 63:2658–2664
Korsching E, Packeisen J, Liedtke C, Hungermann D, Wulfing P, van Diest PJ, Brandt B, Boecker W, Buerger H (2005) The origin of vimentin expression in invasive breast cancer: epithelial–mesenchymal transition, myoepithelial histogenesis or histogenesis from progenitor cells with bilinear differentiation potential? J Pathol 206:451–457
Ashiru O, Boutet P, Fernandez-Messina L, Aguera-Gonzalez S, Skepper JN, Vales-Gomez M, Reyburn HT (2010) Natural killer cell cytotoxicity is suppressed by exposure to the human NKG2D ligand MICA*008 that is shed by tumor cells in exosomes. Cancer Res 70:481–489
Hu J, Qin K, Zhang Y, Gong J, Li N, Lv D, Xiang R, Tan X (2011) Downregulation of transcription factor Oct4 induces an epithelial-to-mesenchymal transition via enhancement of Ca2 + influx in breast cancer cells. Biochem Biophys Res Commun 411:786–791
Tsuda H, Takarabe T, Hasegawa F, Fukutomi T, Hirohashi S (2000) Large, central acellular zones indicating myoepithelial tumor differentiation in high-grade invasive ductal carcinomas as markers of predisposition to lung and brain metastases. Am J Surg Pathol 24:197–202
Hunt NC, Douglas-Jones AG, Jasani B, Morgan JM, Pignatelli M (1997) Loss of E-cadherin expression associated with lymph node metastases in small breast carcinomas. Virchows Arch 430:285–289
Kowalski PJ, Rubin MA, Kleer CG (2003) E-cadherin expression in primary carcinomas of the breast and its distant metastases. Breast Cancer Res 5:R217–R222
Dong HM, Liu G, Wu J, Lu JS, Luo JM, Shen ZZ, Shao ZM (2006) Biological significance of E-cadherin in an inflammatory breast carcinoma cell line. Zhonghua Zhong Liu Za Zhi 28:4–7
Tsuji T, Ibaragi S, Hu GF (2009) Epithelial–mesenchymal transition and cell cooperativity in metastasis. Cancer Res 69:7135–7139
Zhang X, Hashemi SS, Yousefi M, Gao C, Sheng J, Ni J, Wang W, Mason J, Man YG (2009) Atypical E-cadherin expression in cell clusters overlying focally disrupted mammary myoepithelial cell layers: implications for tumor cell motility and invasion. Pathol Res Pract 205:375–385
Chao Y, Wu Q, Acquafondata M, Dhir R, Wells A (2012) Partial mesenchymal to epithelial reverting transition in breast and prostate cancer metastases. Cancer Microenviron 5:19–28
Garber K (2002) Angiogenesis inhibitors suffer new setback. Nat Biotechnol 20:1067–1068
Sakariassen PO, Prestegarden L, Wang J, Skaftnesmo KO, Mahesparan R, Molthoff C, Sminia P, Sundlisaeter E, Misra A, Tysnes BB, Chekenya M, Peters H, Lende G, Kalland KH, Oyan AM, Petersen K, Jonassen I, van der Kogel A, Feuerstein BG, Terzis AJ, Bjerkvig R, Enger PO (2006) Angiogenesis-independent tumor growth mediated by stem-like cancer cells. Proc Natl Acad Sci U S A 103:16466–16471
Song N, Huang Y, Shi H, Yuan S, Ding Y, Song X, Fu Y, Luo Y (2009) Overexpression of platelet-derived growth factor-BB increases tumor pericyte content via stromal-derived factor-1alpha/CXCR4 axis. Cancer Res 69:6057–6064
Wendel C, Hemping-Bovenkerk A, Krasnyanska J, Mees ST, Kochetkova M, Stoeppeler S, Haier J (2012) CXCR4/CXCL12 participate in extravasation of metastasizing breast cancer cells within the liver in a rat model. PLoS ONE 7:e30046
Smith MC, Luker KE, Garbow JR, Prior JL, Jackson E, Piwnica-Worms D, Luker GD (2004) CXCR4 regulates growth of both primary and metastatic breast cancer. Cancer Res 64:8604–8612
da Silva BB, Lopes-Costa PV, dos Santos AR, de Sousa-Junior EC, Alencar AP, Pires CG, Rosal MA (2009) Comparison of three vascular endothelial markers in the evaluation of microvessel density in breast cancer. Eur J Gynaecol Oncol 30:285–288
Chen YT, Chen WT, Huang WT, Wu CC, Chai CY (2012) Expression of MMP-2, MMP-9 and MMP-11 in dermatofibroma and dermatofibrosarcoma protuberans. Kaohsiung J Med Sci 28:545–549
Takahashi RH, Matsubayashi J, Yokotsuka M, Tachibana M, Kusama H, Nagao T (2012) An intrapelvic extraintestinal gastrointestinal stromal tumor of undetermined origin: diagnosis by prostate needle biopsy. Pathol Res Pract 208:736–740
Mao Y, Keller ET, Garfield DH, Shen K, Wang J (2012) Stromal cells in tumor microenvironment and breast cancer. Cancer Metastasis Rev 32(1–2):303–315
Acknowledgments
Study was supported by The Scientific and Technological Research Council of Turkey (TÜBİTAK Grant No: 109S449). Authors thank Prof. Dr. Necdet Demir for his suggestions in interpreting the immunohistochemical staining, and Sayra Dilmaç for her technical assistance.
Conflict of interest
The authors also declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Erin, N., Kale, Ş., Tanrıöver, G. et al. Differential characteristics of heart, liver, and brain metastatic subsets of murine breast carcinoma. Breast Cancer Res Treat 139, 677–689 (2013). https://doi.org/10.1007/s10549-013-2584-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-013-2584-0