Abstract
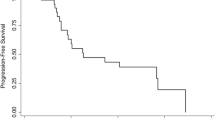
Capecitabine produces an objective response rate of up to 25 % in anthracycline-treated, taxane-resistant metastatic breast cancer (MBC). The farnesyltransferase inhibitor tipifarnib inhibits Ras signaling and has clinical activity when used alone in MBC. The objective of this study was to determine the efficacy and safety of tipifarnib–capecitabine combination in MBC patients who were previously treated with an anthracycline and progressed on taxane therapy. Eligible patients received oral capecitabine 1,000 mg/m2 twice daily plus oral tipifarnib 300 mg twice daily on days 1–14 every 21 days. The primary endpoint was ORR. The trial was powered to detect an improvement in response rate from 25 to 40 %. Among 63 eligible, partial response occurred in six patients (9.5 %; 90 % CI 4.2–17.9 %), median progression-free survival was 2.6 months (95 % CI 2.1–4.4), and median overall survival was 11.4 months (95 % CI 7.7–14.0). Dose modifications were required for 43 patients (68 %) for either tipifarnib and/or capecitabine. Grades 3 and 4 toxicities were seen in 30 patients (44 %; 90 % CI 44.4–67.0 %) and 11 patients (16 %; 90 % CI 10.8–29.0 %), respectively. The most common grade 3 toxicities included neutropenia, nausea, and vomiting; and the most common grade 4 toxicity was neutropenia (8 out of 11 cases). The tipifarnib–capecitabine combination is not more effective than capecitabine alone in MBC patients who were previously treated with an anthracycline and taxane therapy.


Similar content being viewed by others
References
Ishikawa T, Sekiguchi F, Fukase Y et al (1998) Positive correlation between the efficacy of capecitabine and doxifluridine and the ratio of thymidine phosphorylase to dihydropyrimidine dehydrogenase activities in tumors in human cancer xenografts. Cancer Res 58:685–690
Blum JL, Jones SE, Buzdar AU et al (1999) Multicenter phase II study of capecitabine in paclitaxel-refractory metastatic breast cancer. J Clin Oncol 17:485–493
Blum JL, Dieras V, Lo Russo PM et al (2001) Multicenter, phase II study of capecitabine in taxane-pretreated metastatic breast carcinoma patients. Cancer 92:1759–1768
Hennessy BT, Gauthier AM, Michaud LB et al (2005) Lower dose capecitabine has a more favorable therapeutic index in metastatic breast cancer: retrospective analysis of patients treated at MD Anderson Cancer Center and a review of capecitabine toxicity in the literature. Ann Oncol 16:1289–1296
Leonard R, Hennessy BT, Blum JL, O’Shaughnessy J (2011) Dose-adjusting capecitabine minimizes adverse effects while maintaining efficacy: a retrospective review of capecitabine for metastatic breast cancer. Clin Breast Cancer 11:349–356
Downward J (2006) Signal transduction. Prelude to an anniversary for the RAS oncogene. Science 314:433–434
Yue W, Fan P, Wang J et al (2007) Mechanisms of acquired resistance to endocrine therapy in hormone-dependent breast cancer cells. J Steroid Biochem Mol Biol 106:102–110
Berstein LM, Zheng H, Yue W et al (2003) New approaches to the understanding of tamoxifen action and resistance. Endocr Relat Cancer 10:267–277
Eralp Y, Derin D, Ozluk Y et al (2008) MAPK overexpression is associated with anthracycline resistance and increased risk for recurrence in patients with triple-negative breast cancer. Ann Oncol 19:669–674
Kelland LR, Smith V, Valenti M et al (2001) Preclinical antitumor activity and pharmacodynamic studies with the farnesyl protein transferase inhibitor R115777 in human breast cancer. Clin Cancer Res 7:3544–3550
Rowinsky EK, Windle JJ, Von Hoff DD (1999) Ras protein farnesyltransferase: a strategic target for anticancer therapeutic development. J Clin Oncol 17:3631–3652
Li T, Sparano JA (2003) Inhibiting ras signaling in the therapy of breast cancer. Clin Breast Cancer 3:405–416
Appels NM, Beijnen JH, Schellens JH (2005) Development of farnesyl transferase inhibitors: a review. Oncologist 10:565–578
Johnston SR, Hickish T, Ellis P et al (2003) Phase II study of the efficacy and tolerability of two dosing regimens of the farnesyl transferase inhibitor, R115777, in advanced breast cancer. J Clin Oncol 21:2492–2499
Sparano JA, Moulder S, Kazi A et al (2006) Targeted inhibition of farnesyltransferase in locally advanced breast cancer: a phase I and II trial of tipifarnib plus dose-dense doxorubicin and cyclophosphamide. J Clin Oncol 24:3013–3018
Gore L, Holden SN, Cohen RB et al (2006) A phase I safety, pharmacological and biological study of the farnesyl protein transferase inhibitor, tipifarnib and capecitabine in advanced solid tumors. Ann Oncol 17:1709–1717
Therasse P, Arbuck SG, Eisenhauer EA et al (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216
Brookmeyer R, Crowley J (1982) A confidence interval for the medial survival time. Biometrics 38:29–41
Kaplan ELaM P (1958) Nonparametric estimation from incomplete observations. J Am Stat Assoc 53:457–481
Greenwood M (1926) The natural duration of cancer. Reports on Public Health and Medical Subjects, vol 22. Her Majesty’s Stationery Office, London, pp 1–26
Hortobagyi GN, Gomez HL, Li RK et al (2010) Analysis of overall survival from a phase III study of ixabepilone plus capecitabine versus capecitabine in patients with MBC resistant to anthracyclines and taxanes. Breast Cancer Res Treat 122:409–418
Jassem J, Fein L, Karwal M, Campone M, Peck R, Poulart V, Vahdat L (2012) Ixabepilone plus capecitabine in advanced breast cancer patients with early relapse after adjuvant anthracyclines and taxanes: a pooled subset analysis of two phase III studies. Breast 21(1):89–94
Sparano JA, Vrdoljak E, Rixe O et al (2010) Randomized phase III trial of ixabepilone plus capecitabine versus capecitabine in patients with metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol 28:3256–3263
Thomas ES, Gomez HL, Li RK et al (2007) Ixabepilone plus capecitabine for metastatic breast cancer progressing after anthracycline and taxane treatment. J Clin Oncol 25:5210–5217
Johnston SR, Semiglazov VF, Manikhas GM, Spaeth D, Romieu G, Dodwell DJ, Wardley AM, Neven P, Bessems A, Park YC, De Porre PM, Perez Ruixo JJ, Howes AJ (2008) A phase II, randomized, blinded study of the farnesyltransferase inhibitor tipifarnib combined with letrozole in the treatment of advanced breast cancer after antiestrogen therapy. Breast Cancer Res Treat 110(2):327–335
Raponi M, Belly RT, Karp JE et al (2004) Microarray analysis reveals genetic pathways modulated by tipifarnib in acute myeloid leukemia. BMC Cancer 4:56
Raponi M, Harousseau JL, Lancet JE et al (2007) Identification of molecular predictors of response in a study of tipifarnib treatment in relapsed and refractory acute myelogenous leukemia. Clin Cancer Res 13:2254–2260
Acknowledgments
This study was conducted by the ECOG (Robert L. Comis, MD, Chair) and supported in part by Public Health Service Grants CA23318 (to the EGOG statistical center), CA66636 (to the ECOG data management center), CA21115 (to the ECOG coordinating center), CA14958 (to Albert Einstein College of Medicine), CA17145 (to Northwestern University Medical Center), CA49883 (to Indiana University Medical Center), CA25224 (to NCCTG) and from the National Cancer Institute, National Institutes of Health and the Department of Health and Human Services. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute. TL is also supported by UL1 RR024146 from the National Center for Research Resources.
Conflict of interest
All authors disclose any financial and personal relationships with other people or organizations that could inappropriately influence their study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, T., Guo, M., Gradishar, W.J. et al. A phase II trial of capecitabine in combination with the farnesyltransferase inhibitor tipifarnib in patients with anthracycline-treated and taxane-resistant metastatic breast cancer: an Eastern Cooperative Oncology Group Study (E1103). Breast Cancer Res Treat 134, 345–352 (2012). https://doi.org/10.1007/s10549-012-2071-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-012-2071-z