Summary
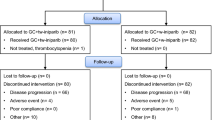
Background Tipifarnib is an orally active, competitive inhibitor of farnesyltransferase which has shown encouraging signs of activity either alone or when combined with other agents. Clinical studies of tipifarnib in combination with anti-estrogen therapy have yielded disappointing results. In contrast, tipifarnib appears to be synergistic in combination with anthracycline based chemotherapy. Here we report the results of the first prospective phase II trial evaluating the efficacy of the novel combination of tipifarnib and gemcitabine in the treatment of metastatic breast cancer. Patients and Methods 30 postmenopausal women with metastatic breast cancer were treated on a 21-day cycle with tipifarnib 300 mg PO twice daily from days 1 through 14. Gemcitabine was administered intravenously at a dose of 1000 mg/m2 on days 1 and 8. Patients were treated until disease progression or unacceptable toxicity. Results There was one complete response and four partial responses yielding an objective response rate of 16.7%. Median progression-free survival and overall survival was 2.5 months (95% confidence interval: 1.6–5.7 months) and 13.1 months (95% confidence interval: 9.1–20.6 months), respectively. 40% of patients experienced grade 4 neutropenia in this study. Conclusion The combination of tipifarnib and gemcitabine is not well tolerated with high rates of myelosuppression and is not more effective than gemcitabine monotherapy in the treatment of metastatic breast cancer.


Similar content being viewed by others
References
End DW, Smets G, Todd AV, Applegate TL, Fuery CJ, Angibaud P, Venet M, Sanz G, Poignet H, Skrzat S, Devine A, Wouters W, Bowden C (2001) Characterization of the antitumor effects of the selective farnesyl protein transferase inhibitor R115777 in vivo and in vitro. Cancer Res 61(1):131–137
Kato K, Cox AD, Hisaka MM, Graham SM, Buss JE, Der CJ (1992) Isoprenoid addition to Ras protein is the critical modification for its membrane association and transforming activity. Proc Natl Acad Sci U S A 89(14):6403–6407
Kelland LR, Smith V, Valenti M, Patterson L, Clarke PA, Detre S, End D, Howes AJ, Dowsett M, Workman P, Johnston SR (2001) Preclinical antitumor activity and pharmacodynamic studies with the farnesyl protein transferase inhibitor R115777 in human breast cancer. Clin Cancer Res 7(11):3544–3550
Izbicka E, Campos D, Carrizales G, Patnaik A (2005) Biomarkers of anticancer activity of R115777 (Tipifarnib, Zarnestra) in human breast cancer models in vitro. Anticancer Res 25(5):3215–3223
Warnberg F, White D, Anderson E, Knox F, Clarke RB, Morris J, Bundred NJ (2006) Effect of a farnesyl transferase inhibitor (R115777) on ductal carcinoma in situ of the breast in a human xenograft model and on breast and ovarian cancer cell growth in vitro and in vivo. Breast Cancer Res 8(2):R21. https://doi.org/10.1186/bcr1395
Caraglia M, Giuberti G, Marra M, Di Gennaro E, Facchini G, Caponigro F, Iaffaioli R, Budillon A, Abbruzzese A (2005) Docetaxel induces p53-dependent apoptosis and synergizes with farnesyl transferase inhibitor r115777 in human epithelial cancer cells. Front Biosci 10:2566–2575
Dalenc F, Giamarchi C, Petit M, Poirot M, Favre G, Faye JC (2005) Farnesyl-transferase inhibitor R115,777 enhances tamoxifen inhibition of MCF-7 cell growth through estrogen receptor dependent and independent pathways. Breast Cancer Res 7(6):R1159–R1167. https://doi.org/10.1186/bcr1357
Martin LA, Head JE, Pancholi S, Salter J, Quinn E, Detre S, Kaye S, Howes A, Dowsett M, Johnston SR (2007) The farnesyltransferase inhibitor R115777 (tipifarnib) in combination with tamoxifen acts synergistically to inhibit MCF-7 breast cancer cell proliferation and cell cycle progression in vitro and in vivo. Mol Cancer Ther 6(9):2458–2467. https://doi.org/10.1158/1535-7163.MCT-06-0452
Balasis ME, Forinash KD, Chen YA, Fulp WJ, Coppola D, Hamilton AD, Cheng JQ, Sebti SM (2011) Combination of farnesyltransferase and Akt inhibitors is synergistic in breast cancer cells and causes significant breast tumor regression in ErbB2 transgenic mice. Clin Cancer Res 17(9):2852–2862. https://doi.org/10.1158/1078-0432.CCR-10-2544
Clark GJ, Der CJ (1995) Aberrant function of the Ras signal transduction pathway in human breast cancer. Breast Cancer Res Treat 35(1):133–144
Johnston SR, Hickish T, Ellis P, Houston S, Kelland L, Dowsett M, Salter J, Michiels B, Perez-Ruixo JJ, Palmer P, Howes A (2003) Phase II study of the efficacy and tolerability of two dosing regimens of the farnesyl transferase inhibitor, R115777, in advanced breast cancer. J Clin Oncol 21(13):2492–2499. https://doi.org/10.1200/JCO.2003.10.064
Li T, Christos PJ, Sparano JA, Hershman DL, Hoschander S, O'Brien K, Wright JJ, Vahdat LT (2009) Phase II trial of the farnesyltransferase inhibitor tipifarnib plus fulvestrant in hormone receptor-positive metastatic breast cancer: New York cancer consortium trial P6205. Ann Oncol 20(4):642–647. https://doi.org/10.1093/annonc/mdn689
Dodwell D, Vergote I (2005) A comparison of fulvestrant and the third-generation aromatase inhibitors in the second-line treatment of postmenopausal women with advanced breast cancer. Cancer Treat Rev 31(4):274–282. https://doi.org/10.1016/j.ctrv.2005.03.009
Ingle JN, Suman VJ, Rowland KM, Mirchandani D, Bernath AM, Camoriano JK, Fishkin PA, Nikcevich DA, Perez EA, North Central Cancer Treatment Group Trial N (2006) Fulvestrant in women with advanced breast cancer after progression on prior aromatase inhibitor therapy: north central cancer treatment group trial N0032. J Clin Oncol 24(7):1052–1056. https://doi.org/10.1200/JCO.2005.04.1053
Perey L, Paridaens R, Hawle H, Zaman K, Nole F, Wildiers H, Fiche M, Dietrich D, Clement P, Koberle D, Goldhirsch A, Thurlimann B (2007) Clinical benefit of fulvestrant in postmenopausal women with advanced breast cancer and primary or acquired resistance to aromatase inhibitors: final results of phase II Swiss Group for Clinical Cancer Research Trial (SAKK 21/00). Ann Oncol 18(1):64–69. https://doi.org/10.1093/annonc/mdl341
Chia S, Gradishar W, Mauriac L, Bines J, Amant F, Federico M, Fein L, Romieu G, Buzdar A, Robertson JF, Brufsky A, Possinger K, Rennie P, Sapunar F, Lowe E, Piccart M (2008) Double-blind, randomized placebo controlled trial of fulvestrant compared with exemestane after prior nonsteroidal aromatase inhibitor therapy in postmenopausal women with hormone receptor-positive, advanced breast cancer: results from EFECT. J Clin Oncol 26(10):1664–1670. https://doi.org/10.1200/JCO.2007.13.5822
Johnston SR, Semiglazov VF, Manikhas GM, Spaeth D, Romieu G, Dodwell DJ, Wardley AM, Neven P, Bessems A, Park YC, De Porre PM, Perez Ruixo JJ, Howes AJ (2008) A phase II, randomized, blinded study of the farnesyltransferase inhibitor tipifarnib combined with letrozole in the treatment of advanced breast cancer after antiestrogen therapy. Breast Cancer Res Treat 110(2):327–335. https://doi.org/10.1007/s10549-007-9726-1
Dalenc F, Doisneau-Sixou SF, Allal BC, Marsili S, Lauwers-Cances V, Chaoui K, Schiltz O, Monsarrat B, Filleron T, Renee N, Malissein E, Meunier E, Favre G, Roche H (2010) Tipifarnib plus tamoxifen in tamoxifen-resistant metastatic breast cancer: a negative phase II and screening of potential therapeutic markers by proteomic analysis. Clin Cancer Res 16(4):1264–1271. https://doi.org/10.1158/1078-0432.CCR-09-1192
Li T, Guo M, Gradishar WJ, Sparano JA, Perez EA, Wang M, Sledge GW (2012) A phase II trial of capecitabine in combination with the farnesyltransferase inhibitor tipifarnib in patients with anthracycline-treated and taxane-resistant metastatic breast cancer: an eastern cooperative oncology group study (E1103). Breast Cancer Res Treat 134(1):345–352. https://doi.org/10.1007/s10549-012-2071-z
Andreopoulou E, Vigoda IS, Valero V, Hershman DL, Raptis G, Vahdat LT, Han HS, Wright JJ, Pellegrino CM, Cristofanilli M, Alvarez RH, Fehn K, Fineberg S, Sparano JA (2013) Phase I-II study of the farnesyl transferase inhibitor tipifarnib plus sequential weekly paclitaxel and doxorubicin-cyclophosphamide in HER2/neu-negative inflammatory carcinoma and non-inflammatory estrogen receptor-positive breast carcinoma. Breast Cancer Res Treat 141(3):429–435. https://doi.org/10.1007/s10549-013-2704-x
Sparano JA, Moulder S, Kazi A, Coppola D, Negassa A, Vahdat L, Li T, Pellegrino C, Fineberg S, Munster P, Malafa M, Lee D, Hoschander S, Hopkins U, Hershman D, Wright JJ, Kleer C, Merajver S, Sebti SM (2009) Phase II trial of tipifarnib plus neoadjuvant doxorubicin-cyclophosphamide in patients with clinical stage IIB-IIIC breast cancer. Clin Cancer Res 15(8):2942–2948. https://doi.org/10.1158/1078-0432.CCR-08-2658
Rha SY, Moon YH, Jeung HC, Kim YT, Sohn JH, Yang WI, Suh CO, Kim GE, Roh JK, Chung HC (2005) Gemcitabine monotherapy as salvage chemotherapy in heavily pretreated metastatic breast cancer. Breast Cancer Res Treat 90(3):215–221. https://doi.org/10.1007/s10549-004-2468-4
Blackstein M, Vogel CL, Ambinder R, Cowan J, Iglesias J, Melemed A (2002) Gemcitabine as first-line therapy in patients with metastatic breast cancer: a phase II trial. Oncology 62(1):2–8
Albain KS, Nag SM, Calderillo-Ruiz G, Jordaan JP, Llombart AC, Pluzanska A, Rolski J, Melemed AS, Reyes-Vidal JM, Sekhon JS, Simms L, O'Shaughnessy J (2008) Gemcitabine plus paclitaxel versus paclitaxel monotherapy in patients with metastatic breast cancer and prior anthracycline treatment. J Clin Oncol 26(24):3950–3957. https://doi.org/10.1200/JCO.2007.11.9362
Chan S, Romieu G, Huober J, Delozier T, Tubiana-Hulin M, Schneeweiss A, Lluch A, Llombart A, du Bois A, Kreienberg R, Mayordomo JI, Anton A, Harrison M, Jones A, Carrasco E, Vaury AT, Frimodt-Moller B, Fumoleau P (2009) Phase III study of gemcitabine plus docetaxel compared with capecitabine plus docetaxel for anthracycline-pretreated patients with metastatic breast cancer. J Clin Oncol 27(11):1753–1760. https://doi.org/10.1200/JCO.2007.15.8485
Siegel-Lakhai WS, Crul M, Zhang S, Sparidans RW, Pluim D, Howes A, Solanki B, Beijnen JH, Schellens JH (2005) Phase I and pharmacological study of the farnesyltransferase inhibitor tipifarnib (Zarnestra, R115777) in combination with gemcitabine and cisplatin in patients with advanced solid tumours. Br J Cancer 93(11):1222–1229. https://doi.org/10.1038/sj.bjc.6602850
Sun J, Blaskovich MA, Knowles D, Qian Y, Ohkanda J, Bailey RD, Hamilton AD, Sebti SM (1999) Antitumor efficacy of a novel class of non-thiol-containing peptidomimetic inhibitors of farnesyltransferase and geranylgeranyltransferase I: combination therapy with the cytotoxic agents cisplatin, Taxol, and gemcitabine. Cancer Res 59(19):4919–4926
Adjei AA, Davis JN, Bruzek LM, Erlichman C, Kaufmann SH (2001) Synergy of the protein farnesyltransferase inhibitor SCH66336 and cisplatin in human cancer cell lines. Clin Cancer Res 7(5):1438–1445
Patnaik A, Eckhardt SG, Izbicka E, Tolcher AA, Hammond LA, Takimoto CH, Schwartz G, McCreery H, Goetz A, Mori M, Terada K, Gentner L, Rybak ME, Richards H, Zhang S, Rowinsky EK (2003) A phase I, pharmacokinetic, and biological study of the farnesyltransferase inhibitor tipifarnib in combination with gemcitabine in patients with advanced malignancies. Clin Cancer Res 9(13):4761–4771
Van Cutsem E, van de Velde H, Karasek P, Oettle H, Vervenne WL, Szawlowski A, Schoffski P, Post S, Verslype C, Neumann H, Safran H, Humblet Y, Perez Ruixo J, Ma Y, Von Hoff D (2004) Phase III trial of gemcitabine plus tipifarnib compared with gemcitabine plus placebo in advanced pancreatic cancer. J Clin Oncol 22(8):1430–1438. https://doi.org/10.1200/JCO.2004.10.112
Schmid P, Akrivakis K, Flath B, Grosse Y, Sezer O, Mergenthaler HG, Possinger K (1999) Phase II trial of gemcitabine as prolonged infusion in metastatic breast cancer. Anti-Cancer Drugs 10(7):625–631
Spielmann M, Llombart-Cussac A, Kalla S, Espie M, Namer M, Ferrero JM, Dieras V, Fumoleau P, Cuvier C, Perrocheau G, Ponzio A, Kayitalire L, Pouillart P (2001) Single-agent gemcitabine is active in previously treated metastatic breast cancer. Oncology 60 (4):303-307. Doi:58524
Brodowicz T, Kostler WJ, Moslinger R, Tomek S, Vaclavik I, Herscovici V, Wiltschke C, Steger GG, Wein W, Seifert M, Kubista E, Zielinski CC (2000) Single-agent gemcitabine as second- and third-line treatment in metastatic breast cancer. Breast 9(6):338–342. https://doi.org/10.1054/brst.2000.0170
Park JY, Kim C, Sohn JH, Kim YT, Rha SY, Jang WI, Kim GE, Chung HC (2002) A phase II study of gemcitabine monotherapy in breast cancer patients refractory to anthracycline and Taxane. Cancer Res Treat 34(4):274–279. https://doi.org/10.4143/crt.2002.34.4.274
Sparano JA, Moulder S, Kazi A, Vahdat L, Li T, Pellegrino C, Munster P, Malafa M, Lee D, Hoschander S, Hopkins U, Hershman D, Wright JJ, Sebti SM (2006) Targeted inhibition of farnesyltransferase in locally advanced breast cancer: a phase I and II trial of tipifarnib plus dose-dense doxorubicin and cyclophosphamide. J Clin Oncol 24(19):3013–3018. https://doi.org/10.1200/JCO.2005.04.9114
Acknowledgments
This work was supported in part by the National Institutes of Health/National Cancer Institute (N01-CM-2011-00039). Tipifarnib was provided by the National Cancer Institute (NCI) under a Clinical Trials Agreement (CTA) with Johnson & Johnson Pharmaceutical Research and Development LLC.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflict of interest
The authors declare no relevant potential conflicts of interest.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Yam, C., Murthy, R.K., Valero, V. et al. A phase II study of tipifarnib and gemcitabine in metastatic breast cancer. Invest New Drugs 36, 299–306 (2018). https://doi.org/10.1007/s10637-018-0564-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-018-0564-2