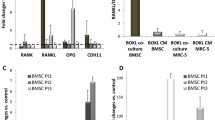
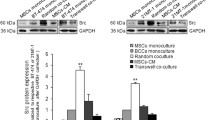
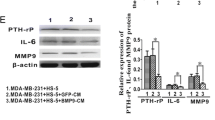
Abstract
The preferential metastasis of breast cancer cells to bone comprises a complex set of events including homing and preferential growth, which may require unique factors produced by bone or other cells in the immediate microenvironment. In this study, an in vitro co-culture system composed of bone mesenchymal stem cells and breast cancer cell lines is used to examine the role of Src kinase on breast cancer cell migration and invasion in the presence of bone-derived cells. This research shows that Src kinase activity in breast cancer cell lines with either high or low levels of endogenous Src activity is increased by bone-derived cell-conditioned medium but not HS68 fibroblast-conditioned medium. Breast cancer cells exhibit enhanced migration in co-culture with bone-derived cells but not HS68 fibroblasts or no co-cultured cells. Inhibition of Src kinase activity using the inhibitors PP2 or saracatinib or using siRNA abrogates the preferential migration of the breast cancer cell lines in response to bone-derived cells. Inhibition of Src activity with saracatinib does not have any significant effect on breast cancer cell invasion in the presence of bone-derived cells. Factors are identified that are produced preferentially by bone-derived cells over HS68 cells that may impact breast cancer cell behavior. This research implicates Src kinase as an important effector of bone-derived cell signals on breast cancer cell migration.





Similar content being viewed by others
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin. doi:10.3322/caac.20107
Coleman RE, Rubens RD (1987) The clinical course of bone metastases from breast cancer. Br J Cancer 55(1):61–66
Coleman RE, Smith P, Rubens RD (1998) Clinical course and prognostic factors following bone recurrence from breast cancer. Br J Cancer 77(2):336–340
Fokas E, Engenhart-Cabillic R, Daniilidis K, Rose F, An HX (2007) Metastasis: the seed and soil theory gains identity. Cancer Metastasis Rev 26(3–4):705–715. doi:10.1007/s10555-007-9088-5
Mundy GR (2002) Metastasis to bone: causes, consequences, and therapeutic opportunities. Nat Rev Cancer 2(8):584–593. doi:10.1038/nrc867
Rabbani SA, Mazar AP (2007) Evaluating distant metastases in breast cancer: from biology to outcomes. Cancer Metastasis Rev 26(3–4):663–674. doi:10.1007/s10555-007-9085-8
Paget S (1889) The distribution of secondary growths in cancer of the breast. Canc Metastasis Rev 8:98
Fidler IJ (1970) Metastasis: guantitative analysis of distribution and fate of tumor embolilabeled with 125 I-5-iodo-2′-deoxyuridine. J Natl Cancer Inst 45(4):773–782
Fidler IJ, Nicolson GL (1977) Fate of recirculating B16 melanoma metastatic variant cells in parabiotic syngeneic recipients. J Natl Cancer Inst 58(6):1867–1872
Klein CA, Seidl S, Petat-Dutter K, Offner S, Geigl JB, Schmidt-Kittler O, Wendler N, Passlick B, Huber RM, Schlimok G, Baeuerle PA, Riethmuller G (2002) Combined transcriptome and genome analysis of single micrometastatic cells. Nat Biotechnol 20(4):387–392. doi:10.1038/nbt0402-387
Morgan TM, Lange PH, Porter MP, Lin DW, Ellis WJ, Gallaher IS, Vessella RL (2009) Disseminated tumor cells in prostate cancer patients after radical prostatectomy and without evidence of disease predicts biochemical recurrence. Clin Cancer Res 15(2):677–683. doi:10.1158/1078-0432.CCR-08-1754
Pantel K, Schlimok G, Braun S, Kutter D, Lindemann F, Schaller G, Funke I, Izbicki JR, Riethmuller G (1993) Differential expression of proliferation-associated molecules in individual micrometastatic carcinoma cells. J Natl Cancer Inst 85(17):1419–1424
Riethdorf S, Wikman H, Pantel K (2008) Review: biological relevance of disseminated tumor cells in cancer patients. Int J Cancer 123(9):1991–2006. doi:10.1002/ijc.23825
Huober J, Thurlimann B (2010) Bone targeted therapy in breast cancer: present and future. Crit Rev Oncol Hematol 74(1):S7–S10. doi:10.1016/S1040-8428(10)70004-4
Rose AA, Siegel PM (2010) Emerging therapeutic targets in breast cancer bone metastasis. Future Oncol 6(1):55–74. doi:10.2217/fon.09.138
Albanese I, Scibetta AG, Migliavacca M, Russo A, Bazan V, Tomasino RM, Colomba P, Tagliavia M, La Farina M (2004) Heterogeneity within and between primary colorectal carcinomas and matched metastases as revealed by analysis of Ki-ras and p53 mutations. Biochem Biophys Res Commun 325(3):784–791. doi:10.1016/j.bbrc.2004.10.111
Gow CH, Chang YL, Hsu YC, Tsai MF, Wu CT, Yu CJ, Yang CH, Lee YC, Yang PC, Shih JY (2009) Comparison of epidermal growth factor receptor mutations between primary and corresponding metastatic tumors in tyrosine kinase inhibitor-naive non-small-cell lung cancer. Ann Oncol 20(4):696–702. doi:10.1093/annonc/mdn679
Stoecklein NH, Klein CA (2010) Genetic disparity between primary tumours, disseminated tumour cells, and manifest metastasis. Int J Cancer 126(3):589–598. doi:10.1002/ijc.24916
Tortola S, Steinert R, Hantschick M, Peinado MA, Gastinger I, Stosiek P, Lippert H, Schlegel W, Reymond MA (2001) Discordance between K-ras mutations in bone marrow micrometastases and the primary tumor in colorectal cancer. J Clin Oncol 19(11):2837–2843
Amir E, Ooi WS, Simmons C, Kahn H, Christakis M, Popovic S, Kalina M, Chesney A, Singh G, Clemons M (2008) Discordance between receptor status in primary and metastatic breast cancer: an exploratory study of bone and bone marrow biopsies. Clin Oncol (R Coll Radiol) 20(10):763–768. doi:10.1016/j.clon.2008.08.005
Broom RJ, Tang PA, Simmons C, Bordeleau L, Mulligan AM, O’Malley FP, Miller N, Andrulis IL, Brenner DM, Clemons MJ (2009) Changes in estrogen receptor, progesterone receptor and Her-2/neu status with time: discordance rates between primary and metastatic breast cancer. Anticancer Res 29(5):1557–1562
Koro K, Parkin S, Pohorelic B, Yang AD, Narendran A, Egan C, Magliocco A (2010) Interactions between breast cancer cells and bone marrow derived cells in vitro define a role for osteopontin in affecting breast cancer cell migration. Breast Cancer Res Treat. doi:10.1007/s10549-010-0889-9
Wheeler DL, Iida M, Dunn EF (2009) The role of Src in solid tumors. Oncologist 14(7):667–678. doi:10.1634/theoncologist.2009-0009
Kim LC, Song L, Haura EB (2009) Src kinases as therapeutic targets for cancer. Nat Rev Clin Oncol 6(10):587–595. doi:10.1038/nrclinonc.2009.129
Morgan L, Nicholson RI, Hiscox S (2008) SRC as a therapeutic target in breast cancer. Endocr Metab Immune Disord Drug Targets 8(4):273–278
Hilbig A (2008) Src kinase and pancreatic cancer. Recent Results Cancer Res 177:179–185
Chang YM, Bai L, Liu S, Yang JC, Kung HJ, Evans CP (2008) Src family kinase oncogenic potential and pathways in prostate cancer as revealed by AZD0530. Oncogene 27(49):6365–6375. doi:10.1038/onc.2008.250
Planas-Silva MD, Bruggeman RD, Grenko RT, Stanley Smith J (2006) Role of c-Src and focal adhesion kinase in progression and metastasis of estrogen receptor-positive breast cancer. Biochem Biophys Res Commun 341(1):73–81. doi:10.1016/j.bbrc.2005.12.164
Hiscox S, Morgan L, Green TP, Barrow D, Gee J, Nicholson RI (2006) Elevated Src activity promotes cellular invasion and motility in tamoxifen resistant breast cancer cells. Breast Cancer Res Treat 97(3):263–274
Hiscox S, Morgan L, Green T, Nicholson RI (2006) Src as a therapeutic target in anti-hormone/anti-growth factor-resistant breast cancer. Endocr Relat Cancer 13(1):S53–S59
Myoui A, Nishimura R, Williams PJ, Hiraga T, Tamura D, Michigami T, Mundy GR, Yoneda T (2003) C-SRC tyrosine kinase activity is associated with tumor colonization in bone and lung in an animal model of human breast cancer metastasis. Cancer Res 63(16):5028–5033
Frame MC (2002) Src in cancer: deregulation and consequences for cell behaviour. Biochim Biophys Acta 1602(2):114–130
Zambuzzi WF, Milani R, Teti A (2010) Expanding the role of Src and protein tyrosine phosphatases balance in modulating osteoblast metabolism: lessons from mice. Biochimie. doi:10.1016/j.biochi.2010.01.002
Zambuzzi WF, Milani R, Teti A (2010) Expanding the role of Src and protein-tyrosine phosphatases balance in modulating osteoblast metabolism: lessons from mice. Biochimie 92(4):327–332. doi:10.1016/j.biochi.2010.01.002
de Vries TJ, Mullender MG, van Duin MA, Semeins CM, James N, Green TP, Everts V, Klein-Nulend J (2009) The Src inhibitor AZD0530 reversibly inhibits the formation and activity of human osteoclasts. Mol Cancer Res 7(4):476–488. doi:10.1158/1541-7786.MCR-08-0219
Zou W, Kitaura H, Reeve J, Long F, Tybulewicz VL, Shattil SJ, Ginsberg MH, Ross FP, Teitelbaum SL (2007) Syk, c-Src, the alphavbeta3 integrin, and ITAM immunoreceptors, in concert, regulate osteoclastic bone resorption. J Cell Biol 176(6):877–888. doi:10.1083/jcb.200611083
Miyazaki T, Sanjay A, Neff L, Tanaka S, Horne WC, Baron R (2004) Src kinase activity is essential for osteoclast function. J Biol Chem 279(17):17660–17666
Liu X, Feng R (2010) Inhibition of epithelial to mesenchymal transition in metastatic breast carcinoma cells by c-Src suppression. Acta Biochim Biophys Sin (Shanghai) 42(7):496–501. doi:10.1093/abbs/gmq043
Zhang XH, Wang Q, Gerald W, Hudis CA, Norton L, Smid M, Foekens JA, Massague J (2009) Latent bone metastasis in breast cancer tied to Src-dependent survival signals. Cancer Cell 16(1):67–78. doi:10.1016/j.ccr.2009.05.017
Sgroi DC (2009) Breast cancer SRC activity: bad to the bone. Cancer Cell 16(1):1–2. doi:10.1016/j.ccr.2009.06.010
Saad F, Lipton A (2010) SRC kinase inhibition: targeting bone metastases and tumor growth in prostate and breast cancer. Cancer Treat Rev 36(2):177–184. doi:10.1016/j.ctrv.2009.11.005
Hiscox S, Barrett-Lee P, Borley AC, Nicholson RI (2010) Combining Src inhibitors and aromatase inhibitors: a novel strategy for overcoming endocrine resistance and bone loss. Eur J Cancer. doi:10.1016/j.ejca.2010.04.012
Araujo J, Logothetis C (2010) Dasatinib: a potent SRC inhibitor in clinical development for the treatment of solid tumors. Cancer Treat Rev. doi: 10.1016/j.ctrv.2010.02.015
Schweppe RE, Kerege AA, French JD, Sharma V, Grzywa RL, Haugen BR (2009) Inhibition of Src with AZD0530 reveals the Src-focal adhesion kinase complex as a novel therapeutic target in papillary and anaplastic thyroid cancer. J Clin Endocrinol Metab 94(6):2199–2203. doi:10.1210/jc.2008-2511
Rajeshkumar NV, Tan AC, De Oliveira E, Womack C, Wombwell H, Morgan S, Warren MV, Walker J, Green TP, Jimeno A, Messersmith WA, Hidalgo M (2009) Antitumor effects and biomarkers of activity of AZD0530, a Src inhibitor, in pancreatic cancer. Clin Cancer Res 15(12):4138–4146. doi:10.1158/1078-0432.CCR-08-3021
Purnell PR, Mack PC, Tepper CG, Evans CP, Green TP, Gumerlock PH, Lara PN, Gandara DR, Kung HJ, Gautschi O (2009) The Src inhibitor AZD0530 blocks invasion and may act as a radiosensitizer in lung cancer cells. J Thorac Oncol 4(4):448–454. doi:10.1097/JTO.0b013e31819c78fb
Rucci N, Susa M, Teti A (2008) Inhibition of protein kinase c-Src as a therapeutic approach for cancer and bone metastases. Anticancer Agents Med Chem 8(3):342–349
Hiscox S, Nicholson RI (2008) Src inhibitors in breast cancer therapy. Expert Opin Ther Targets 12(6):757–767. doi:10.1517/14728222.12.6.757
Lee D, Gautschi O (2006) Clinical development of SRC tyrosine kinase inhibitors in lung cancer. Clin Lung Cancer 7(6):381–384
Zheng R, Yano S, Matsumori Y, Nakataki E, Muguruma H, Yoshizumi M, Sone S (2005) SRC tyrosine kinase inhibitor, m475271, suppresses subcutaneous growth and production of lung metastasis via inhibition of proliferation, invasion, and vascularization of human lung adenocarcinoma cells. Clin Exp Metastasis 22(3):195–204
Bagrodia S, Chackalaparampil I, Kmiecik TE, Shalloway D (1991) Altered tyrosine 527 phosphorylation and mitotic activation of p60c-src. Nature 349(6305):172–175
Bjorge JD, Jakymiw A, Fujita DJ (2000) Selected glimpses into the activation and function of Src kinase. Oncogene 19(49):5620–5635
Bjorge JD, O’Connor TJ, Fujita DJ (1996) Activation of human pp60c-src. Biochem Cell Biol 74(4):477–484
Cartwright CA, Kamps MP, Meisler AI, Pipas JM, Eckhart W (1989) pp60c-src activation in human colon carcinoma. J Clin Invest 83(6):2025–2033. doi:10.1172/JCI114113
Cooper JA, Howell B (1993) The when and how of Src regulation. Cell 73(6):1051–1054
Guarino M (2010) Src signaling in cancer invasion. J Cell Physiol 223(1):14–26. doi:10.1002/jcp.22011
Superti-Furga G, Courtneidge SA (1995) Structure–function relationships in Src family and related protein tyrosine kinases. Bioessays 17(4):321–330
Green TP, Fennell M, Whittaker R, Curwen J, Jacobs V, Allen J, Logie A, Hargreaves J, Hickinson DM, Wilkinson RW, Elvin P, Boyer B, Carragher N, Ple PA, Bermingham A, Holdgate GA, Ward WH, Hennequin LF, Davies BR, Costello GF (2009) Preclinical anticancer activity of the potent, oral Src inhibitor AZD0530. Mol Oncol. doi:10.1016/j.molonc.2009.01.002
Previdi S, Maroni P, Matteucci E, Broggini M, Bendinelli P, Desiderio MA (2010) Interaction between human-breast cancer metastasis and bone microenvironment through activated hepatocyte growth factor/Met and beta-catenin/Wnt pathways. Eur J Cancer 46(9):1679–1691. doi:10.1016/j.ejca.2010.02.036
Coskun U, Gunel N, Sancak B, Gunel U, Onuk E, Bayram O, Yilmaz E, Candan S, Ozkan S (2003) Significance of serum vascular endothelial growth factor, insulin-like growth factor-I levels and nitric oxide activity in breast cancer patients. Breast 12(2):104–110
Coskun U, Gunel N, Toruner FB, Sancak B, Onuk E, Bayram O, Cengiz O, Yilmaz E, Elbeg S, Ozkan S (2003) Serum leptin, prolactin and vascular endothelial growth factor (VEGF) levels in patients with breast cancer. Neoplasma 50(1):41–46
Ahmed OI, Adel AM, Diab DR, Gobran NS (2006) Prognostic value of serum level of interleukin-6 and interleukin-8 in metastatic breast cancer patients. Egypt J Immunol 13(2):61–68
Bendre MS, Gaddy-Kurten D, Mon-Foote T, Akel NS, Skinner RA, Nicholas RW, Suva LJ (2002) Expression of interleukin 8 and not parathyroid hormone-related protein by human breast cancer cells correlates with bone metastasis in vivo. Cancer Res 62(19):5571–5579
Benoy IH, Salgado R, Van Dam P, Geboers K, Van Marck E, Scharpe S, Vermeulen PB, Dirix LY (2004) Increased serum interleukin-8 in patients with early and metastatic breast cancer correlates with early dissemination and survival. Clin Cancer Res 10(21):7157–7162. doi:10.1158/1078-0432.CCR-04-0812
Salgado R, Junius S, Benoy I, Van Dam P, Vermeulen P, Van Marck E, Huget P, Dirix LY (2003) Circulating interleukin-6 predicts survival in patients with metastatic breast cancer. Int J Cancer 103(5):642–646. doi:10.1002/ijc.10833
Sasser AK, Sullivan NJ, Studebaker AW, Hendey LF, Axel AE, Hall BM (2007) Interleukin-6 is a potent growth factor for ER-alpha-positive human breast cancer. FASEB J 21(13):3763–3770. doi:10.1096/fj.07-8832com
Zhang GJ, Adachi I (1999) Serum interleukin-6 levels correlate to tumor progression and prognosis in metastatic breast carcinoma. Anticancer Res 19(2B):1427–1432
Chan PC, Chen YL, Cheng CH, Yu KC, Cary LA, Shu KH, Ho WL, Chen HC (2003) Src phosphorylates Grb2-associated binder 1 upon hepatocyte growth factor stimulation. J Biol Chem 278(45):44075–44082. doi:10.1074/jbc.M305745200
Crostella L, Lidder S, Williams R, Skouteris GG (2001) Hepatocyte growth factor/scatter factor-induces phosphorylation of cortactin in A431 cells in a Src kinase-independent manner. Oncogene 20(28):3735–3745. doi:10.1038/sj.onc.1204474
Matteucci E, Ridolfi E, Maroni P, Bendinelli P, Desiderio MA (2007) c-Src/histone deacetylase 3 interaction is crucial for hepatocyte growth factor dependent decrease of CXCR4 expression in highly invasive breast tumor cells. Mol Cancer Res 5(8):833–845. doi:10.1158/1541-7786.MCR-07-0054
Rahimi N, Hung W, Tremblay E, Saulnier R, Elliott B (1998) c-Src kinase activity is required for hepatocyte growth factor-induced motility and anchorage-independent growth of mammary carcinoma cells. J Biol Chem 273(50):33714–33721
Duval M, Le Boeuf F, Huot J, Gratton JP (2007) Src-mediated phosphorylation of Hsp90 in response to vascular endothelial growth factor (VEGF) is required for VEGF receptor-2 signaling to endothelial NO synthase. Mol Biol Cell 18(11):4659–4668. doi:10.1091/mbc.E07-05-0467
Eliceiri BP, Puente XS, Hood JD, Stupack DG, Schlaepfer DD, Huang XZ, Sheppard D, Cheresh DA (2002) Src-mediated coupling of focal adhesion kinase to integrin alpha(v)beta5 in vascular endothelial growth factor signaling. J Cell Biol 157(1):149–160. doi:10.1083/jcb.200109079
Lesslie DP, Summy JM, Parikh NU, Fan F, Trevino JG, Sawyer TK, Metcalf CA, Shakespeare WC, Hicklin DJ, Ellis LM, Gallick GE (2006) Vascular endothelial growth factor receptor-1 mediates migration of human colorectal carcinoma cells by activation of Src family kinases. Br J Cancer 94(11):1710–1717. doi:10.1038/sj.bjc.6603143
Guy CT, Muthuswamy SK, Cardiff RD, Soriano P, Muller WJ (1994) Activation of the c-Src tyrosine kinase is required for the induction of mammary tumors in transgenic mice. Genes Dev 8(1):23–32
Muthuswamy SK, Muller WJ (1994) Activation of the Src family of tyrosine kinases in mammary tumorigenesis. Adv Cancer Res 64:111–123
Muthuswamy SK, Siegel PM, Dankort DL, Webster MA, Muller WJ (1994) Mammary tumors expressing the neu proto-oncogene possess elevated c-Src tyrosine kinase activity. Mol Cell Biol 14(1):735–743
Wilson GR, Cramer A, Welman A, Knox F, Swindell R, Kawakatsu H, Clarke RB, Dive C, Bundred NJ (2006) Activated c-SRC in ductal carcinoma in situ correlates with high tumour grade, high proliferation and HER2 positivity. Br J Cancer 95(10):1410–1414. doi:10.1038/sj.bjc.6603444
Zou D, Yoon HS, Anjomshoaa A, Perez D, Fukuzawa R, Guilford P, Humar B (2009) Increased levels of active c-Src distinguish invasive from in situ lobular lesions. Breast Cancer Res 11 (4):R45. doi:10.1186/bcr2332
Boyer B, Bourgeois Y, Poupon MF (2002) Src kinase contributes to the metastatic spread of carcinoma cells. Oncogene 21(15):2347–2356. doi:10.1038/sj.onc.1205298
Edwards J (2010) Src kinase inhibitors: an emerging therapeutic treatment option for prostate cancer. Expert Opin Investig Drugs 19(5):605–614. doi:10.1517/13543781003789388
Elsberger B, Stewart B, Tatarov O, Edwards J (2010) Is Src a viable target for treating solid tumours? Curr Cancer Drug Targets 10(7):683–694
Onishi T, Hayashi N, Theriault RL, Hortobagyi GN, Ueno NT (2010) Future directions of bone-targeted therapy for metastatic breast cancer. Nat Rev Clin Oncol 7(11):641–651. doi:10.1038/nrclinonc.2010.134
Eissa SA, Zaki SA, El-Maghraby SM, Kadry DY (2005) Importance of serum IL-18 and RANTES as markers for breast carcinoma progression. J Egypt Natl Canc Inst 17(1):51–55
Azenshtein E, Luboshits G, Shina S, Neumark E, Shahbazian D, Weil M, Wigler N, Keydar I, Ben-Baruch A (2002) The CC chemokine RANTES in breast carcinoma progression: regulation of expression and potential mechanisms of promalignant activity. Cancer Res 62(4):1093–1102
Dimberg J, Hugander A, Wagsater D (2006) Protein expression of the chemokine, CCL28, in human colorectal cancer. Int J Oncol 28(2):315–319
Kawai Y, Kaidoh M, Yokoyama Y, Sano K, Ohhashi T (2009) Chemokine CCL2 facilitates ICAM-1-mediated interactions of cancer cells and lymphatic endothelial cells in sentinel lymph nodes. Cancer Sci 100(3):419–428. doi:10.1111/j.1349-7006.2008.01064.x
Kroeze KL, Jurgens WJ, Doulabi BZ, van Milligen FJ, Scheper RJ, Gibbs S (2009) Chemokine-mediated migration of skin-derived stem cells: predominant role for CCL5/RANTES. J Invest Dermatol 129(6):1569–1581. doi:10.1038/jid.2008.405
Luboshits G, Shina S, Kaplan O, Engelberg S, Nass D, Lifshitz-Mercer B, Chaitchik S, Keydar I, Ben-Baruch A (1999) Elevated expression of the CC chemokine regulated on activation, normal T cell expressed and secreted (RANTES) in advanced breast carcinoma. Cancer Res 59(18):4681–4687
Makinoshima H, Dezawa M (2009) Pancreatic cancer cells activate CCL5 expression in mesenchymal stromal cells through the insulin-like growth factor-I pathway. FEBS Lett 583(22):3697–3703. doi:10.1016/j.febslet.2009.10.061
Soria G, Yaal-Hahoshen N, Azenshtein E, Shina S, Leider-Trejo L, Ryvo L, Cohen-Hillel E, Shtabsky A, Ehrlich M, Meshel T, Keydar I, Ben-Baruch A (2008) Concomitant expression of the chemokines RANTES and MCP-1 in human breast cancer: a basis for tumor-promoting interactions. Cytokine 44(1):191–200. doi:10.1016/j.cyto.2008.08.002
Wigler N, Shina S, Kaplan O, Luboshits G, Chaitchik S, Keydar I, Ben-Baruch A (2002) Breast carcinoma: a report on the potential usage of the CC chemokine RANTES as a marker for a progressive disease. Isr Med Assoc J 4(11):940–943
Wu Y, Li YY, Matsushima K, Baba T, Mukaida N (2008) CCL3-CCR5 axis regulates intratumoral accumulation of leukocytes and fibroblasts and promotes angiogenesis in murine lung metastasis process. J Immunol 181(9):6384–6393
Hoar FJ, Chaudhri S, Wadley MS, Stonelake PS (2003) Co-expression of vascular endothelial growth factor C (VEGF-C) and c-erbB2 in human breast carcinoma. Eur J Cancer 39(12):1698–1703
Linderholm B, Grankvist K, Wilking N, Johansson M, Tavelin B, Henriksson R (2000) Correlation of vascular endothelial growth factor content with recurrences, survival, and first relapse site in primary node-positive breast carcinoma after adjuvant treatment. J Clin Oncol 18(7):1423–1431
Ryden L, Jirstrom K, Haglund M, Stal O, Ferno M (2010) Epidermal growth factor receptor and vascular endothelial growth factor receptor 2 are specific biomarkers in triple-negative breast cancer. Results from a controlled randomized trial with long-term follow-up. Breast Cancer Res Treat 120 (2):491–498. doi:10.1007/s10549-010-0758-6
Jedeszko C, Victor BC, Podgorski I, Sloane BF (2009) Fibroblast hepatocyte growth factor promotes invasion of human mammary ductal carcinoma in situ. Cancer Res 69(23):9148–9155. doi:10.1158/0008-5472.CAN-09-1043
Mine S, Fujisaki T, Kawahara C, Tabata T, Iida T, Yasuda M, Yoneda T, Tanaka Y (2003) Hepatocyte growth factor enhances adhesion of breast cancer cells to endothelial cells in vitro through up-regulation of CD44. Exp Cell Res 288(1):189–197
Mukhopadhyay D, Tsiokas L, Zhou XM, Foster D, Brugge JS, Sukhatme VP (1995) Hypoxic induction of human vascular endothelial growth factor expression through c-Src activation. Nature 375(6532):577–581
Acknowledgments
The authors would like to thank Lynn Feasel and the staff at the Rockyview General Hospital Clinical Orthopaedics Department for their assistance in obtaining and processing bone samples. They would also like to thank Elizabeth Kornaga for help with the statistical analysis. Alberta Cancer Board, Breast Cancer Operating Grant Project #23141. Canadian Breast Cancer Foundation—Prairies/NWT Chapter, Project “Development of Diagnostic and Therapeutic Agents that recognize a Mutated Form of Src Kinase in Breast Cancer”.
Conflicts of interest
None of the authors have a conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pohorelic, B., Singh, R., Parkin, S. et al. Role of Src in breast cancer cell migration and invasion in a breast cell/bone-derived cell microenvironment. Breast Cancer Res Treat 133, 201–214 (2012). https://doi.org/10.1007/s10549-011-1753-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-011-1753-2