Abstract
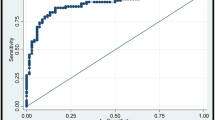
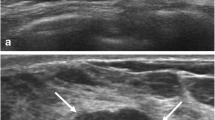
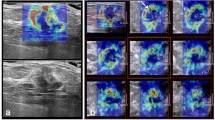
Shear wave elastography (SWE) is an emerging technique which can obtain quantitative elasticity values in breast disease. We therefore evaluated the diagnostic performance of SWE for the differentiation of breast masses compared with conventional ultrasound (US). Conventional US and SWE were performed by three experienced radiologists for 158 consecutive women who had been scheduled for US-guided core biopsy or surgical excision in 182 breast masses (89 malignancies and 93 benign; mean size, 1.76 cm). For each lesion, quantitative elasticity was measured in terms of the Young’s modulus (in kilopascals, kPa) with SWE, and BI-RADS final categories were assessed with conventional US. The mean elasticity values were significantly higher in malignant masses (153.3 kPa ± 58.1) than in benign masses (46.1 kPa ± 42.9), (P < 0.0001). The average mean elasticity values of invasive ductal (157.5 ± 57.07) or invasive lobular (169.5 ± 61.06) carcinomas were higher than those of ductal carcinoma in situ (117.8 kPa ± 54.72). The average mean value was 49.58 ± 43.51 for fibroadenoma, 35.3 ± 31.2 for fibrocystic changes, 69.5 ± 63.2 for intraductal papilloma, and 149.5 ± 132.4 for adenosis or stromal fibrosis. The optimal cut-off value, yielding the maximal sum of sensitivity and specificity, was 80.17 kPa, and the sensitivity and specificity of SWE were 88.8% (79 of 89) and 84.9% (79 of 93). The area under the ROC curve (Az value) was 0.898 for conventional US, 0.932 for SWE, and 0.982 for combined data. In conclusion, there were significant differences in the elasticity values of benign and malignant masses as well as invasive and intraductal cancers with SWE. Our results suggest that SWE has the potential to aid in the differentiation of benign and malignant breast lesions.








Similar content being viewed by others
References
Itoh A, Ueno E, Tohno E et al (2006) Breast disease: clinical application of US elastography for diagnosis. Radiology 239:341–350
Fleury EdFC, Fleury JC, Piato S, Roveda D Jr (2009) New elastographic classification of breast lesions during and after compression. Diagn Interv Radiol 15:96–103
Burnside ES, Hall TJ et al (2007) Differentiating benign from malignant solid breast masses with US strain imaging. Radiology 245:401–410
Regner DM, Hesley GK, Hangiandreou NJ et al (2006) Breast lesions: evaluation with US strain imaging-clinical experience of multiple observers. Radiology 238:425–437
Athanasiou A, Tardivon A, Tanter M et al (2010) Breast lesions: quantitative elastography with supersonic shear imaging—preliminary results. Radiology 256:297–303
Bercoff J, Tanter M, Fink M (2004) Supersonic shear imaging: a new technique for soft tissue elasticity mapping. IEEE Trans Ultrason Ferroelectr Freq Control 51:396–409
Bercoff J, Tanter M, Muller M, Fink M (2004) The role of viscosity in the impulse diffraction field of elastic waves induced by the acoustic radiation force. IEEE Trans Ultrason Ferroelectr Freq Control 51:1523–1536
Bercoff J, Chaffai S, Tanter M et al (2003) In vivo breast tumor detection using transient elastography. Ultrasound Med Biol 29:1387–1396
Tanter M, Bercoff J, Athanasiou A et al (2008) Quantitative assessment of breast lesion viscoelasticity: initial clinical results using supersonic shear imaging. Ultrasound Med Biol 34:1373–1386
Mendelson EB, Baum JK, Berg WA, Merritt CRB, Rubin E (2003) Breast Imaging Reporting and Data System, BI-RADS: Ultrasound, 1st edn. American College of Radiology, Reston
Heinze G (2004) A SAS macro, S-PLUS library and R package to perform logistic regression without convergence problems. Technical Report 2/2004: Medical University of Vienna, Vienna
Evans A, Whelehan P, Thomson K et al (2010) Quantitative shear wave ultrasound elastography: initial experience in solid breast masses. Breast Cancer Res 12:R104
Raza S, Odulate A, Ong EM, Chikarmane S, Harston CW (2010) Using real-time tissue elastography for breast lesion evaluation: our initial experience. J Ultrasound Med 29:551–563
Scaperrotta G, Ferranti C, Costa C et al (2008) Role of sonoelastography in non-palpable breast lesions. Eur Radiol 18:2381–2389
Thomas A, Fischer T, Frey H et al (2006) Real-time elastography—an advanced method of ultrasound: first results in 108 patients with breast lesions. Ultrasound Obstet Gynecol 28:335–340
Cho N, Moon WK, Park JS, Cha JH, Jang M, Seong MH (2008) Nonpalpable breast masses: evaluation by US elastography. Korean J Radiol 9:111–118
Egorov V, Kearney T, Pollak SB et al (2009) Differentiation of benign and malignant breast lesions by mechanical imaging. Breast Cancer Res Treat 118:67–80
Chung SY, Moon WK, Choi JW, Cho N, Jang M, Kim KG (2010) Differentiation of benign from malignant nonpalpable breast masses: a comparison of computer-assisted quantification and visual assessment of lesion stiffness with the use of sonographic elastography. Acta Radiol 51:9–14
Chang JM, Moon WK, Cho N, Kim SJ (2011) Breast mass evaluation: factors influencing the quality of US elastography. Radiology 259:59–64
Acknowledgment
This study was supported by a grant from the Innovative Research Institute for Cell Therapy (A062260) and by a grant from the National R&D Program for Cancer Control (A01185), Ministry of Health & Welfare, Republic of Korea. The authors appreciated the statistical advice from the Medical Research Collaborating Center at the Seoul National University Hospital and the Seoul National University College of Medicine.
Conflict of interest
Authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chang, J.M., Moon, W.K., Cho, N. et al. Clinical application of shear wave elastography (SWE) in the diagnosis of benign and malignant breast diseases. Breast Cancer Res Treat 129, 89–97 (2011). https://doi.org/10.1007/s10549-011-1627-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-011-1627-7