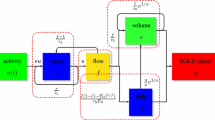
Abstract
In many physiological or pathological situations, the interpretation of BOLD signals remains elusive as the intimate link between neuronal activity and subsequent flow/metabolic changes is not fully understood. During the past decades, a number of biophysical models of the neurovascular coupling have been proposed. It is now well-admitted that these models may bridge between observations (fMRI data) and underlying biophysical and (patho-)physiological mechanisms (related to flow and metabolism) by providing mechanistic explanations. In this study, three well-established models (Buxton’s, Friston’s and Sotero’s) are investigated. An exhaustive parameter sensitivity analysis (PSA) was conducted to study the marginal and joint influences of model parameters on the three main features of the BOLD response (namely the principal peak, the post-stimulus undershoot and the initial dip). In each model, parameters that have the greatest (and least) influence on the BOLD features as well as on the direction of variation of these features were identified. Among the three studied models, parameters were shown to affect the output features in different manners. Indeed, the main parameters revealed by the PSA were found to strongly depend on the way the flow(CBF)-metabolism(CMRO2) relationship is implemented (serial vs. parallel). This study confirmed that the model structure which accounts for the representation of the CBF–CMRO2 relationship (oxygen supply by the flow vs. oxygen demand from neurons) plays a key role. More generally, this work provides substantial information about the tuning of parameters in the three considered models and about the subsequent interpretation of BOLD signals based on these models.





Similar content being viewed by others
References
Arthurs OJ, Boniface S (2002) How well do we understand the neural origins of the fMRI BOLD signal? Trends Neurosci 25(1):27–31
Aubert A, Costalat R (2002) A model of the coupling between brain electrical activity, metabolism, and hemodynamics: application to the interpretation of functional neuroimaging. Neuroimage 17(3):1162–1181
Buxton RB (2001) The elusive initial dip. Neuroimage 13(6 Pt 1):953–958
Buxton RB, Frank LR (1997) A model for the coupling between cerebral blood flow and oxygen metabolism during neural stimulation. J Cereb Blood Flow Metab 17(1):64–72
Buxton RB, Wong EC, Frank LR (1998) Dynamics of blood flow and oxygenation changes during brain activation: the balloon model. Magn Reson Med 39(6):855–864
Buxton RB, Uludag K, Dubowitz DJ, Liu TT (2004) Modeling the hemodynamic response to brain activation. Neuroimage 23(Suppl 1):S220–S233
Daunizeau J, Grova C, Mattout J, Marrelec G, Clonda D, Goulard B, Pelegrini-Issac M, Lina JM, Benali H (2005) Assessing the relevance of fMRI-based prior in the EEG inverse problem: a Bayesian model comparison approach. IEEE Trans Sign Proc 53(9):3461–3472
Davis TL, Kwong KK, Weisskoff RM, Rosen BR (1998) Calibrated functional MRI: mapping the dynamics of oxidative metabolism. Proc Natl Acad Sci USA 95(4):1834–1839
Deneux T, Faugeras O (2006) Using nonlinear models in fMRI data analysis: model selection and activation detection. Neuroimage 32(4):1669–1689
Fieberg J, Jenkins KJ (2005) Assessing uncertainty in ecological systems using global sensitivity analyses: a case example of simulated wolf reintroduction effects on elk. Ecol Modell 187(2–3):259–280
Frahm J, Baudewig J, Kallenberg K, Kastrup A, Merboldt KD, Dechent P (2008) The post-stimulation undershoot in BOLD fMRI of human brain is not caused by elevated cerebral blood volume. Neuroimage 40(2):473–481
Friston KJ, Mechelli A, Turner R, Price CJ (2000) Nonlinear responses in fMRI: the Balloon model, Volterra kernels, and other hemodynamics. Neuroimage 12(4):466–477
Grubb RL Jr, Raichle ME, Eichling JO, Ter-Pogossian MM (1974) The effects of changes in PaCO2 on cerebral blood volume, blood flow, and vascular mean transit time. Stroke 5(5):630–639
Herman P, Sanganahalli BG, Hyder F (2009) Multimodal measurements of blood plasma and red blood cell volumes during functional brain activation. J Cereb Blood Flow Metab 29(1):19–24
Horwitz B, Poeppel D (2002) How can EEG/MEG and fMRI/PET data be combined? Hum Brain Mapp 17(1):1–3
Hu Z, Shi P (2007) Nonlinear analysis of BOLD signal: biophysical modeling, physiological states, and functional activation. Med Image Comput Comput Assist Interv 10(Pt 2):734–741
Jacobsen DJ, Hansen LK, Madsen KH (2008) Bayesian model comparison in nonlinear BOLD fMRI hemodynamics. Neural Comput 20(3):738–755
Jolivet R, Magistretti PJ, Weber B (2009) Deciphering neuron-glia compartmentalization in cortical energy metabolism. Front Neuroenergetics 1:4
Lee SP, Duong TQ, Yang G, Iadecola C, Kim SG (2001) Relative changes of cerebral arterial and venous blood volumes during increased cerebral blood flow: implications for BOLD fMRI. Magn Reson Med 45(5):791–800
Li G, Wang SW, Rabitz H, Wang S, Jaffé P (2002) Global uncertainty assessments by high dimensional model representations (HDMR). Chem Eng Sci 57(21):4445–4460
Li G, Rabitz H, Wang SW, Georgopoulos PG (2003) Correlation method for variance reduction of Monte Carlo integration in RS-HDMR. J Comput Chem 24(3):277–283
Liu AK, Belliveau JW, Dale AM (1998) Spatiotemporal imaging of human brain activity using functional MRI constrained magnetoencephalography data: Monte Carlo simulations. Proc Natl Acad Sci USA 95(15):8945–8950
Mandeville JB, Marota JJ, Ayata C, Zaharchuk G, Moskowitz MA, Rosen BR, Weisskoff RM (1999) Evidence of a cerebrovascular postarteriole windkessel with delayed compliance. J Cereb Blood Flow Metab 19(6):679–689
Nunez PL, Silberstein RB (2000) On the relationship of synaptic activity to macroscopic measurements: does co-registration of EEG with fMRI make sense? Brain Topogr 13(2):79–96
Obata T, Liu TT, Miller KL, Luh WM, Wong EC, Frank LR, Buxton RB (2004) Discrepancies between BOLD and flow dynamics in primary and supplementary motor areas: application of the balloon model to the interpretation of BOLD transients. Neuroimage 21(1):144–153
Rabitz H, Alis OF (1999) General foundations of high-dimensional model representations. J Math Chem 25(2–3):197–233
Saltelli A (2002) Making best use of model evaluations to compute sensitivity indices. Comput Phys Commun 145(2):280–297
Schroeter ML, Kupka T, Mildner T, Uludag K, von Cramon DY (2006) Investigating the post-stimulus undershoot of the BOLD signal—a simultaneous fMRI and fNIRS study. Neuroimage 30(2):349–358
Shulman RG, Rothman DL, Hyder F (2007) A BOLD search for baseline. Neuroimage 36(2):277–281
Sobol’ IM (2001) Global sensitivity indices for nonlinear mathematical models and their Monte Carlo estimates. Math Comput Simul 55(1–3):271–280
Sotero RC, Trujillo-Barreto NJ (2007) Modelling the role of excitatory and inhibitory neuronal activity in the generation of the BOLD signal. Neuroimage 35(1):149–165
Sotero RC, Trujillo-Barreto NJ (2008) Biophysical model for integrating neuronal activity, EEG, fMRI and metabolism. Neuroimage 39(1):290–309
Sotero RC, Trujillo-Barreto NJ, Jimenez JC, Carbonell F, Rodriguez-Rojas R (2009) Identification and comparison of stochastic metabolic/hemodynamic models (sMHM) for the generation of the BOLD signal. J Comput Neurosci 26(2):251–269
Stephan KE, Weiskopf N, Drysdale PM, Robinson PA, Friston KJ (2007) Comparing hemodynamic models with DCM. Neuroimage 38(3):387–401
Toga AW, Mazziotta JC (2002) Brain mapping: the methods, 2nd edn. Academic Press
Vazquez AL, Cohen ER, Gulani V, Hernandez-Garcia L, Zheng Y, Lee GR, Kim SG, Grotberg JB, Noll DC (2006) Vascular dynamics and BOLD fMRI: CBF level effects and analysis considerations. Neuroimage 32(4):1642–1655
Zheng Y, Martindale J, Johnston D, Jones M, Berwick J, Mayhew J (2002) A model of the hemodynamic response and oxygen delivery to brain. Neuroimage 16(3 Pt 1):617–637
Ziehn T, Tomlin AS (2009) GUI-HDMR—a software tool for global sensitivity analysis of complex models. Environ Modell Softw 24(7):775–785
Acknowledgments
This work was supported (i) by INSERM (collaborative project Inserm-Inria, Institute “Technologies de la Santé”, 2008–2010) as a 2-year post-doc position for SB and (ii) by ANR Blanc 2010 (MULTIMODEL Project). The authors would like to thank Tilo Ziehn for providing us with the GUI-HDMR toolbox. They are also grateful to the two anonymous reviewers for helpful comments on an earlier version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Appendix A
Appendix A
In contrast to local approaches (like derivative-based methods) which provide results around relevant “working points”, variance-based methods can deal with extended ranges of parameters (Sobol’ 2001).
These methods are based on the ANOVA-decomposition of the output (features) variance. Assuming that any feature extracted from simulated BOLD signals (typically A type or D type , type standing for dip, peak or under) is a nonlinear function Φ of an unknown vector of parameters θ, the ANOVA-decomposition (Rabitz and Alis 1999; Sobol’ 2001) of Φ can be written as
If Φ0 = ∫Φ(θ)dθ represents the mean value and if \( \int\nolimits_{0}^{1} {\Upphi_{{i_{1} \ldots i_{s}}} d_{{\theta_{{i_{p}}}}} = 0} \) for 1 ≤ p ≤ s and 1 ≤ s ≤ n and 1 ≤ i 1 <···< i s ≤ n, then the expression of Eq. A.1 is defined in a unique way. As explained in (Li et al. 2002), the first order component function Φ i (θ i ) in Eq. A.1 represents the independent contribution of the ith input parameter θ i to the feature Φ(θ). The second order component function Φ ij (θ i , θ j ) represents the joint contribution of the parameter pair (θ i , θ j ) to the considered feature and so on until the last term which contains the residuals at the nth order (joint contribution of all input parameters). The marginal and joint variances (at 2nd order) are given by
The total variance of the output Φ(θ) is given by σ T = ∫Φ2(θ)dθ − Φ 20 . Starting from the exact expansion of Eq. A.1 and the previous variances, one can theoretically calculate the relative variance of each contribution with respect to the total variance σ T of the output:
The sensitivity indices S i and S ij characterize respectively the marginal influence of parameter θ i and the joint influence of parameters θ i and θ j on the feature of interest. In practice, the total variance is easy to compute, but the computation of the many integrals involved in the marginal variances gets very difficult when increasing the number of input parameters. Improvements can be done by using ad-hoc screening methods (Monte Carlo methods in (Saltelli 2002; Sobol’ 2001)) in order to maximize the number of samples without increasing too much the resulting computational cost. In the purpose of reducing the sampling effort, (Rabitz and Alis 1999) proposed an alternative approach which approximates the output by some projections functions φ r (that can be seen as a metamodel representing the output) before computing the sensitivity indices. For example using a polynomial representation for the projection functions, it can be shown that Φ i and Φi,j can be expressed as polynomials of the projection functions
Later, (Li et al. 2002) improved the method showing that in this case of polynomial approximation, one can have a direct access to the marginal and joint variances \( \sigma_{i} \approx \sum\nolimits_{r = 1}^{k} {(\alpha_{r}^{i} )^{2} } \) and \( \sigma_{ij} \approx \sum\nolimits_{p = 1}^{l} {} \sum\nolimits_{q = 1}^{m} {\left( {\beta_{pq}^{ij} } \right)^{2} } \).
Rights and permissions
About this article
Cite this article
Blanchard, S., Papadopoulo, T., Bénar, CG. et al. Relationship Between Flow and Metabolism in BOLD Signals: Insights from Biophysical Models. Brain Topogr 24, 40–53 (2011). https://doi.org/10.1007/s10548-010-0166-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10548-010-0166-6