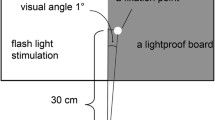
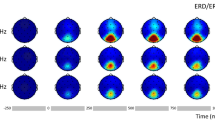
Abstract
The sensitivity of visual areas to different temporal frequencies, as well as the functional connections between these areas, was examined using magnetoencephalography (MEG). Alternating circular sinusoids (0, 3.1, 8.7 and 14 Hz) were presented to foveal and peripheral locations in the visual field to target ventral and dorsal stream structures, respectively. It was hypothesized that higher temporal frequencies would preferentially activate dorsal stream structures. To determine the effect of frequency on the cortical response we analyzed the late time interval (220–770 ms) using a multi-dipole spatio-temporal analysis approach to provide source locations and timecourses for each condition. As an exploratory aspect, we performed cross-correlation analysis on the source timecourses to determine which sources responded similarly within conditions. Contrary to predictions, dorsal stream areas were not activated more frequently during high temporal frequency stimulation. However, across cortical sources the frequency-following response showed a difference, with significantly higher power at the second harmonic for the 3.1 and 8.7 Hz stimulation and at the first and second harmonics for the 14 Hz stimulation with this pattern seen robustly in area V1. Cross-correlations of the source timecourses showed that both low- and high-order visual areas, including dorsal and ventral stream areas, were significantly correlated in the late time interval. The results imply that frequency information is transferred to higher-order visual areas without translation. Despite the less complex waveforms seen in the late interval of time, the cross-correlation results show that visual, temporal and parietal cortical areas are intricately involved in late-interval visual processing.
Similar content being viewed by others
References
1 Ahlfors, S.P., Simpson, G.V., Dale, A.M., Belliveau, J.W., Liu, A.K., Korvenoja, A., Virtanen, J., Huotilainen, M., Tootell, R.B., Aronen, H.J. and Ilmoniemi, R.J. Spatiotemporal activity of a cortical network for processing visual motion revealed by MEG and fMRI. J. Neurophysiol., 1999, 82(5): 2545–2555.
2 Ahonen, A.I., Hä mä lä inen, M.S., Kajola, M.J., Knuutila, J.E.T., Laine, P.L., Lounasmaa, O.V., Simola, J.T., Tesche, C.D. and Vilkman, V.A. A 122-channel magnetometer covering the whole head. Proceedings of the Satellite Symposium on Neuroscience and Technology, 14th Annual Conference of the IEEE Eng. Med. Bio. Soc., 1992.
3 Aine, C., Huang, M., Stephen, J. and Christner, R. Multistart algorithms for MEG empirical data analysis reliably characterize locations and time courses of multiple sources. NeuroImage, 2000, 12(2): 159–172.
4 Aine, C.J., Stephen, J.M., Christner, R., Hudson, D. and Best, E. Task relevance enhances early transient and late slow-wave activity of distributed cortical sources. J. Comput. Neurosci., 2003, 15(2): 203–221.
5 Aine, C.J., Supek, S. and George, J.S. Temporal dynamics of visual-evoked neuromagnetic sources: Effects of stimulus parameters and selective attention. Int. J. Neurosci., 1995, 80(1–4): 79–104.
6 Aine, C.J., Supek, S., George, J.S., Ranken, D., Lewine, J., Sanders, J., Best, E., Tiee, W., Flynn, E.R. and Wood, C.C. Retinotopic organization of human visual cortex: Departures from the classical model. Cereb. Cortex, 1996, 6(3): 354–361.
7 Arakawa, K., Tobimatsu, S., Tomoda, H., Kira, J. and Kato, M. The effect of spatial frequency on chromatic and achromatic steady-state visual evoked potentials. Clin. Neurophysiol., 1999, 110(11): 1959–1964.
8 Baizer, J.S., Ungerleider, L.G. and Desimone, R. Organization of visual inputs to the inferior temporal and posterior parietal cortex in macaques. J. Neurosci., 1991, 11(1): 168–190.
9 Bijl, G.K. The visual electrically evoked potential (VEEP): Steady-state responses. Electroencephalogr. Clin. Neurophysiol., 1984, 57(3): 264–269.
10 Boussaoud, D., Desimone, R. and Ungerleider, L.G. Visual topography of area TEO in the macaque. J. Comp. Neurol., 1991, 306(4): 554–575.
11 Boussaoud, D., Ungerleider, L.G. and Desimone, R. Pathways for motion analysis: Cortical connections of the medial superior temporal and fundus of the superior temporal visual areas in the macaque. J. Comp. Neurol., 1990, 296(3): 462–495.
12 Breitmeyer, B.G. and Ganz, L. Implications of sustained and transient channels for theories of visual pattern masking, saccadic suppression, and information processing. Psychol. Rev., 1976, 83(1): 1–36.
13 Diamond, A.L. Latency of the steady state visual evoked potential. Electroencephalogr. Clin. Neurophysiol., 1977, 42(1): 125–127.
14 Endo, S., Toyama, H., Kimura, Y., Ishii, K., Senda, M., Kiyosawa, M. and Uchiyama, A. Mapping visual field with positron emission tomography by mathematical modeling of the retinotopic organization in the calcarine cortex. IEEE Trans. Med. Imaging, 1997, 16(3): 252–260.
15 Engel, A.K., Konig, P., Kreiter, A.K., Schillen, T.B. and Singer, W. Temporal coding in the visual cortex: New vistas on integration in the nervous system. Trends Neurosci., 1992, 15(6): 218–226.
16 Engel, A.K., Konig, P., Kreiter, A.K. and Singer, W. Interhemispheric synchronization of oscillatory neuronal responses in cat visual cortex. Science, 1991, 252(5010): 1177–1179.
17 Enroth-Cugell, C. and Robson, J. The contrast sensitivity of retinal ganglion cells of the cat. J. Physiol., 1966, 187: 517–551.
18 Fawcett, I.P., Barnes, G.R., Hillebrand, A. and Singh, K.D. The temporal frequency tuning of human visual cortex investigated using synthetic aperture magnetometry. Neuroimage, 2004, 21(4): 1542–1553.
19 Felleman, D.J. and Van Essen, D.C. Distributed hierarchical processing in the primate cerebral cortex. Cereb. Cortex, 1991, 1(1): 1–47.
20 Ferrera, V.P., Nealey, T.A. and Maunsell, J.H. Responses in macaque visual area V4 following inactivation of the parvocellular and magnocellular LGN pathways. J. Neurosci., 1994, 14(4): 2080–2088.
21 Ferster, D. and LeVay, S. The axonal arborizations of lateral geniculate neurons in the striate cortex of the cat. J. Comp. Neurol., 1978, 182(4 Pt 2): 923–944.
22 Fox, P.T. and Raichle, M.E. Stimulus rate determines regional brain blood flow in striate cortex. Ann. Neurol., 1985, 17(3): 303–305.
23 Gray, C.M. The temporal correlation hypothesis of visual feature integration: Still alive and well. Neuron, 1999, 24(1): 31–47, 111–125.
24 Guy, C.N., Ffytche, D.H., Brovelli, A. and Chumillas, J. fMRI and EEG responses to periodic visual stimulation. NeuroImage, 1999, 10(2): 125–148.
25 Herrmann, C.S. Human EEG responses to 1–100/ Hz flicker: Resonance phenomena in visual cortex and their potential correlation to cognitive phenomena. Exp. Brain Res., 2001, 137(3–4): 346–353.
26 Hilgetag, C.C., O'Neill, M.A. and Young, M.P. Indeterminate organization of the visual system. Science, 1996a, 271(5250): 776–777.
27 Hilgetag, C.-C., O'Neill, M.A. and Young, M.P. Indeterminate organization of the visual system. Science, 1996b, 271: 776–777.
28 Hou, C., Pettet, M.W., Sampath, V., Candy, T.R. and Norcia, A.M. Development of the spatial organization and dynamics of lateral interactions in the human visual system. J. Neurosci., 2003, 23(25): 8630–8640.
29 Huang, M., Aine, C.J., Supek, S., Best, E., Ranken, D. and Flynn, E.R. Multi-start downhill simplex method for spatio-temporal source localization in magnetoencephalography. Electroencephalogr. Clin. Neurophysiol., 1998, 108(1): 32–44.
30 Huang, M.X., Aine, C., Davis, L., Butman, J., Christner, R., Weisend, M., Stephen, J., Meyer, J., Silveri, J., Herman, M. and Lee, R.R. Sources on the anterior and posterior banks of the central sulcus identified from magnetic somatosensory evoked responses using multistart spatio-temporal localization. Hum. Brain Mapp., 2000, 11(2): 59–76.
31 Huk, A.C. and Heeger, D.J. Task-related modulation of visual cortex. J. Neurophysiol., 2000, 83(6): 3525–3536.
32 Klemm, W.R., Gibbons, W.D., Allen, R.G. and Harrison, J.M. Differences among humans in steady-state evoked potentials: Evaluation of alpha activity, attentiveness and cognitive awareness of perceptual effectiveness. Neuropsychologia, 1982, 20(3): 317–325.
33 Klemm, W.R., Gibbons, W.D., Allen, R.G. and Richey, E.O. Hemispheric lateralization and handedness correlation of human evoked “steady-state” responses to patterned visual stimuli. Physiol. Psychol., 1980, 8(3): 409–416.
34 Klemm, W.R., Goodson, R.A. and Allen, R.G. Steady-state visual evoked responses in anesthetized monkeys. Brain. Res. Bull., 1984, 13(2): 287–291.
35 Levitt, J.B., Kiper, D.C. and Movshon, J.A. Receptive fields and functional architecture of macaque V2. J. Neurophysiol., 1994a, 71(6): 2517–2542.
36 Levitt, J.B., Yoshioka, T. and Lund, J.S. Intrinsic cortical connections in macaque visual area V2: Evidence for interaction between different functional streams. J. Comp. Neurol., 1994b, 342(4): 551–570.
37 Liavas, A.P., Moustakides, G.V., Henning, G., Psarakis, E.Z. and Husar, P. A periodogram-based method for the detection of steady-state visually evoked potentials. IEEE Trans. Biomed. Eng., 1998, 45(2): 242–248.
38 Livingstone, M.S. and Hubel, D.H. Psychophysical evidence for separate channels for the perception of form, color, movement, and depth. J. Neurosci., 1987, 7(11): 3416–3468.
39 Malonek, D., Tootell, R.B. and Grinvald, A. Optical imaging reveals the functional architecture of neurons processing shape and motion in owl monkey area MT. Proc. Biol. Sci, 1994, 258(1352): 109–119.
40 Mast, J. and Victor, J.D. Fluctuations of steady-state VEPs: Interaction of driven evoked potentials and the EEG. Electroencephalogr. Clin. Neurophysiol., 1991, 78(5): 389–401.
41 Merigan, W.H. Chromatic and achromatic vision of macaques: Role of the P pathway. J. Neurosci., 1989, 9(3): 776–783.
42 Merigan, W.H. and Maunsell, J.H. How parallel are the primate visual pathways? Annu. Rev. Neurosci., 1993, 16: 369–402.
43 Moradi, F., Liu, L.C., Cheng, K., Waggoner, R.A., Tanaka, K. and Ioannides, A.A. Consistent and precise localization of brain activity in human primary visual cortex by MEG and fMRI. NeuroImage, 2003, 18(3): 595–609.
44 Muller, M.M., Picton, T.W., Valdes-Sosa, P., Riera, J., Teder-Salejarvi, W.A. and Hillyard, S.A. Effects of spatial selective attention on the steady-state visual evoked potential in the 20–28/ Hz range. Cogn. Brain Res., 1998, 6(4): 249–261.
45 Muller, M.M., Teder, W. and Hillyard, S.A. Magnetoencephalographic recording of steady-state visual evoked cortical activity. Brain Topogr., 1997, 9(3): 163–168.
46 Nakayama, K. and Mackeben, M. Steady state visual evoked potentials in the alert primate. Vision Res., 1982, 22(10): 1261–1271.
47 Okada, Y.C., Kaufman, L., Brenner, D. and Williamson, S.J. Modulation transfer functions of the human visual system revealed by magnetic field measurements. Vision Res., 1982, 22(2): 319–333.
48 Rager, G. and Singer, W. The response of cat visual cortex to flicker stimuli of variable frequency. Eur. J. Neurosci., 1998, 10(5): 1856–1877.
49 Regan, D. Some characteristics of average steady-state and transient responses evoked by modulated light. Electroencephalogr. Clin. Neurophysiol., 1966, 20(3): 238–248.
50 Regan, D. Chromatic adaptation and steady-state evoked potentials. Vision Res., 1968, 8(2): 149–158.
51 Roelfsema, P.R., Engel, A.K., Konig, P. and Singer, W. Visuomotor integration is associated with zero time-lag synchronization among cortical areas. Nature, 1997, 385(6612): 157–161.
52 Rovamo, J. and Virsu, V. An estimation and application of the human cortical magnification factor. Exp. Brain Res., 1979, 37(3): 495–510.
53 Schiller, P.H., Logothetis, N.K. and Charles, E.R. Functions of the colour-opponent and broad-band channels of the visual system. Nature, 1990, 343(6253): 68–70.
54 Schiller, P.H. and Malpeli, J.G. Functional specificity of lateral geniculate nucleus laminae of the rhesus monkey. J. Neurophysiol., 1978, 41(3): 788–797.
55 Seki, K., Nakasato, N., Fujita, S., Hatanaka, K., Kawamura, T., Kanno, A. and Yoshimoto, T. Neuromagnetic evidence that the P100 component of the pattern reversal visual evoked response originates in the bottom of the calcarine fissure. Electroencephalogr. Clin. Neurophysiol., 1996, 100(5): 436–442.
56 Shigeto, H., Tobimatsu, S., Yamamoto, T., Kobayashi, T. and Kato, M. Visual evoked cortical magnetic responses to checkerboard pattern reversal stimulation: A study on the neural generators of N75, P100 and N145. J. Neurol. Sci., 1998, 156(2): 186–194.
57 Shoham, D., Hubener, M., Schulze, S., Grinvald, A. and Bonhoeffer, T. Spatio-temporal frequency domains and their relation to cytochrome oxidase staining in cat visual cortex. Nature, 1997, 385: 529–532.
58 Simon, F. The phase of PVEP in Maxwellian view: Influence of contrast, spatial and temporal frequency. Vision Res., 1992, 32(4): 591–599.
59 Singer, W. Neuronal synchrony: A versatile code for the definition of relations? Neuron, 1999, 24(1): 49–65, 111–125.
60 Singer, W. and Gray, C.M. Visual feature integration and the temporal correlation hypothesis. Annu. Rev. Neurosci., 1995, 18: 555–586.
61 Spileers, W., Maes, H., Lagae, L. and Orban, G.A. Contrast modulated steady-state visual evoked potentials (CMSS VEPs): Recording evoked potentials and related single cell responses in area 17 of the cat. Electroencephalogr. Clin. Neurophysiol., 1994, 92(1): 64–77.
62 Srinivasan, R., Russell, D.P., Edelman, G.M. and Tononi, G. Increased synchronization of neuromagnetic responses during conscious perception. J. Neurosci., 1999, 19(13): 5435–5448.
63 Stephen, J.M., Aine, C.J., Christner, R.F., Ranken, D., Huang, M. and Best, E. Central versus peripheral visual field stimulation results in timing differences in dorsal stream sources as measured with MEG. Vision Res., 2002, 42(28): 3059–3074.
64 Tardif, E., Bergeron, A., Lepore, F. and Guillemot, J.P. Spatial and temporal frequency tuning and contrast sensitivity of single neurons in area 21a of the cat. Brain Res., 1996, 716(1–2): 219–223.
65 Theiler, J., Eubank, S., Longtin, A., Galdrikian, B. and Farmer, J. Testing for nonlinearity in time series — The method of Surrogate Data. Physica D, 1992, 58(1–4): 77–94.
66 Thomas, C.G. and Menon, R.S. Amplitude response and stimulus presentation frequency response of human primary visual cortex using BOLD EPI at 4 T. Magn. Reson. Med., 1998, 40(2): 203–209.
67 Tobimatsu, S. and Kato, M. The effect of binocular stimulation on each component of transient and steady-state VEPs. Electroencephalogr. Clin. Neurophysiol., 1996, 100(3): 177–183.
68 Tobimatsu, S., Tomoda, H. and Kato, M. Normal variability of the amplitude and phase of steady-state VEPs. Electroencephalogr. Clin. Neurophysiol., 1996, 100(3): 171–176.
69 Tomoda, Y., Tobimatsu, S. and Mitsudome, A. Visual evoked potentials in school children: A comparative study of transient and steady-state methods with pattern reversal and flash stimulation. Clin. Neurophysiol., 1999, 110(1): 97–102.
70 Tononi, G., Srinivasan, R., Russell, D.P. and Edelman, G.M. Investigating neural correlates of conscious perception by frequency-tagged neuromagnetic responses. Proc. Natl. Acad. Sci. USA, 1998, 95(6): 3198–3203.
71 Tootell, R.B., Reppas, J.B., Kwong, K.K., Malach, R., Born, R.T., Brady, T.J., Rosen, B.R. and Belliveau, J.W. Functional analysis of human MT and related visual cortical areas using magnetic resonance imaging. J. Neurosci., 1995, 15(4): 3215–3230.
72 Ungerleider, L. and Mishkin, M. Two cortical visual systems. In: D. Ingle, R. Mansfield and M. Goodale (Eds.), The analysis of visual behavior. MIT Press, Cambridge, 1982: 549–586.
73 Ungerleider, L.G. and Desimone, R. Projections to the superior temporal sulcus from the central and peripheral field representations of V1 and V2. J. Comp. Neurol., 1986, 248(2): 147–163.
74 Vafaee, M.S., Marrett, S., Meyer, E., Evans, A.C. and Gjedde, A. Increased oxygen consumption in human visual cortex: Response to visual stimulation. Acta Neurol. Scand., 1998, 98(2): 85–89.
75 Vafaee, M.S., Meyer, E., Marrett, S., Paus, T., Evans, A.C. and Gjedde, A. Frequency-dependent changes in cerebral metabolic rate of oxygen during activation of human visual cortex. J. Cereb. Blood Flow Metab., 1999, 19(3): 272–277.
76 Van Der Tweel, L.H. and Lunel, H.F. Human Visual Responses to Sinusoidally Modulated Light. Electroencephalogr. Clin. Neurophysiol., 1965, 18: 587–598.
77 Van Essen, D. Functional organization of primate visual cortex. Cereb. Cortex, 1985, 3: 259–329.
78 Van Essen, D. and Maunsell, J.H. Hierarchical organization and functional streams in the visual cortex. Trends Neurosci., 1983, 6: 370–375.
79 Vezoli, J., Falchier, A., Jouve, B., Knoblauch, K., Young, M. and Kennedy, H. Quantitative analysis ofconnectivity in the visual cortex: Extracting function from structure. Neuroscientist, 2004, 10(5): 476–482.
80 Wright, M. and Ikeda, H. Processing of spatial and temporal information in the visual system. In: F. Schmitt and F. Worden (Eds.), The neurosciences (Third Study Program). MIT Press, Cambridge, 1974: 115–122.
81 Zeki, S. The response properties of cells in the middle temporal area (area MT) of owl monkey visual cortex. Proc. R Soc. Lond. B Biol. Sci., 1980, 207(1167): 239–248.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stephen, J.M., Ranken, D.F. & Aine, C.J. Frequency-Following and Connectivity of Different Visual Areas in Response to Contrast-Reversal Stimulation. Brain Topogr 18, 257–272 (2006). https://doi.org/10.1007/s10548-006-0004-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10548-006-0004-z