Abstract
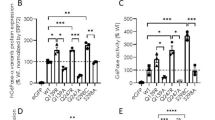
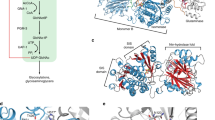
Mutations in genes encoding metabolic enzymes are often the cause of inherited diseases. Mutations usually affect the ability of proteins to fold properly, thus leading to enzyme loss of function. In this work, we explored the relationships between protein stability, aggregation, and degradation in vitro and inside cells in a large set of mutants associated with human phosphoglycerate kinase 1 (hPGK1) deficiency. To this end, we studied a third of the pathogenic alleles reported in the literature using expression analyses and biochemical, biophysical, and computational procedures. Our results show that most pathogenic variants studied had an increased tendency to aggregate when expressed in Escherichia coli, well correlating with the denaturation half-lives measured by thermal denaturation in vitro. Further, the most deleterious mutants show reduced stability toward chemical denaturation and proteolysis, supporting a pivotal role of thermodynamic stability in the propensity toward aggregation and proteolysis of pathogenic hPGK1 mutants in vitro and inside cells. Our strategy allowed us to unravel the complex relationships between protein stability, aggregation, and degradation in hPGK1 deficiency, which might be used to understand disease mechanisms in many inborn errors of metabolism. Our results suggest that pharmacological chaperones and protein homeostasis modulators could be considered as good candidates for therapeutic approaches for hPGK1 deficiency.




Similar content being viewed by others
References
Beutler E (2007) PGK deficiency. Br J Haematol 136(1):3–11
Borghi L, Meschi T, Amato F, Briganti A, Novarini A, Giannini A (1996) Urinary volume, water and recurrences in idiopathic calcium nephrolithiasis: a 5-year randomized prospective study. J Urol 155(3):839–843
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Chiarelli LR, Morera SM, Bianchi P et al (2012) Molecular insights on pathogenic effects of mutations causing phosphoglycerate kinase deficiency. PLoS One 7(2):e32065
Cliff MJ, Bowler MW, Varga A et al (2010) Transition state analogue structures of human phosphoglycerate kinase establish the importance of charge balance in catalysis. J Am Chem Soc 132(18):6507–6516
Fermo E, Bianchi P, Chiarelli LR et al (2012) A new variant of phosphoglycerate kinase deficiency (p.I371K) with multiple tissue involvement: molecular and functional characterization. Mol Genet Metab 106(4):455–461
Gomes CM (2012) Protein misfolding in disease and small molecule therapies. Curr Top Med Chem 12(22):2460–2469
Gregersen N, Bross P, Vang S, Christensen JH (2006) Protein misfolding and human disease. Annu Rev Genomics Hum Genet 7:103–124
Hartl FU, Bracher A, Hayer-Hartl M (2011) Molecular chaperones in protein folding and proteostasis. Nature 475(7356):324–332
Kim YE, Hipp MS, Bracher A, Hayer-Hartl M, Hartl FU (2013) Molecular chaperone functions in protein folding and proteostasis. Annu Rev Biochem 82:323–355
Maeda M, Yoshida A (1991) Molecular defect of a phosphoglycerate kinase variant (PGK-Matsue) associated with hemolytic anemia: Leu—Pro substitution caused by T/A—C/G transition in exon 3. Blood 77(6):1348–1352
Maeda M, Bawle EV, Kulkarni R, Beutler E, Yoshida A (1992) Molecular abnormalities of a phosphoglycerate kinase variant generated by spontaneous mutation. Blood 79(10):2759–2762
Martinez A, Calvo AC, Teigen K, Pey AL (2008) Rescuing proteins of low kinetic stability by chaperones and natural ligands phenylketonuria, a case study. Prog Mol Biol Transl Sci 83:89–134
McCarrey JR, Thomas K (1987) Human testis-specific PGK gene lacks introns and possesses characteristics of a processed gene. Nature 326(6112):501–505
Mesa-Torres N, Fabelo-Rosa I, Riverol D et al (2013) The role of protein denaturation energetics and molecular chaperones in the aggregation and mistargeting of mutants causing primary hyperoxaluria type I. PLoS One 8(8):e71963
Morimoto A, Ueda I, Hirashima Y et al (2003) A novel missense mutation (1060G –> C) in the phosphoglycerate kinase gene in a Japanese boy with chronic haemolytic anaemia, developmental delay and rhabdomyolysis. Br J Haematol 122(6):1009–1013
Noel N, Flanagan JM, Ramirez Bajo MJ et al (2006) Two new phosphoglycerate kinase mutations associated with chronic haemolytic anaemia and neurological dysfunction in two patients from Spain. Br J Haematol 132(4):523–529
Ookawara T, Dave V, Willems P et al (1996) Retarded and aberrant splicings caused by single exon mutation in a phosphoglycerate kinase variant. Arch Biochem Biophys 327(1):35–40
Pey AL (2013a) The interplay between protein stability and dynamics in conformational diseases: the case of hPGK1 deficiency. Biochim Biophys Acta 1834(12):2502–2511
Pey AL (2013b) Protein homeostasis disorders of key enzymes of amino acids metabolism: mutation-induced protein kinetic destabilization and new therapeutic strategies. Amino Acids 45(6):1331–1341
Pey AL, Stricher F, Serrano L, Martinez A (2007) Predicted effects of missense mutations on native-state stability account for phenotypic outcome in phenylketonuria, a paradigm of misfolding diseases. Am J Hum Genet 81(5):1006–1024
Pey AL, Mesa-Torres N, Chiarelli LR, Valentini G (2013) Structural and energetic basis of protein hinetic destabilization in human phosphoglycerate kinase 1 deficiency. Biochemistry 52(7):1160–1170
Powers ET, Morimoto RI, Dillin A, Kelly JW, Balch WE (2009) Biological and chemical approaches to diseases of proteostasis deficiency. Annu Rev Biochem 78:959–991
Raimondi S, Guglielmi F, Giorgetti S et al (2011) Effects of the known pathogenic mutations on the aggregation pathway of the amyloidogenic peptide of apolipoprotein A-I. J Mol Biol 407(3):465–476
Sanchez-Ruiz JM, Lopez-Lacomba JL, Cortijo M, Mateo PL (1988) Differential scanning calorimetry of the irreversible thermal denaturation of thermolysin. Biochemistry 27(5):1648–1652
Sotiriou E, Greene P, Krishna S, Hirano M, DiMauro S (2010) Myopathy and parkinsonism in phosphoglycerate kinase deficiency. Muscle Nerve 41(5):707–710
Spiegel R, Gomez EA, Akman HO, Krishna S, Horovitz Y, DiMauro S (2009) Myopathic form of phosphoglycerate kinase (PGK) deficiency: a new case and pathogenic considerations. Neuromuscul Disord 19(3):207–211
Stefani M (2004) Protein misfolding and aggregation: new examples in medicine and biology of the dark side of the protein world. Biochim Biophys Acta 1739(1):5–25
Tartaglia GG, Pawar AP, Campioni S, Dobson CM, Chiti F, Vendruscolo M (2008) Prediction of aggregation-prone regions in structured proteins. J Mol Biol 380(2):425–436
Tsolis AC, Papandreou NC, Iconomidou VA, Hamodrakas SJ (2013) A consensus method for the prediction of ‘aggregation-prone’ peptides in globular proteins. PLoS One 8(1):e54175
Valentini G, Maggi M, Pey AL (2013) Protein stability, folding and misfolding in human PGK1 deficiency. Biomolecules 3(4):1030–1052
Vendruscolo M, Knowles TP, Dobson CM (2011) Protein solubility and protein homeostasis: a generic view of protein misfolding disorders. Cold Spring Harb Perspect Biol 3(12)
Willard HF, Goss SJ, Holmes MT, Munroe DL (1985) Regional localization of the phosphoglycerate kinase gene and pseudogene on the human X chromosome and assignment of a related DNA sequence to chromosome 19. Hum Genet 71(2):138–143
Acknowledgments
We thank Dr. Jose Manuel Sanchez-Ruiz for support. ALP acknowledges support from MINECO (grants CSD2009-00088 and BIO2012-34937), Junta de Andalucia (grant CTS-11-07187) and FEDER Funds. GV acknowledges support from Ministero dell’Istruzione, dell’Università e della Ricerca - “Fondo per le Agevolazioni alla Ricerca” (FAR). ALP is recipient of a Ramon y Cajal contract from MINECO (RYC2009-04147).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: Niels Gregersen
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 7.50 MB)
Rights and permissions
About this article
Cite this article
Pey, A.L., Maggi, M. & Valentini, G. Insights into human phosphoglycerate kinase 1 deficiency as a conformational disease from biochemical, biophysical, and in vitro expression analyses. J Inherit Metab Dis 37, 909–916 (2014). https://doi.org/10.1007/s10545-014-9721-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10545-014-9721-8