Abstract
The organic acidurias d-2-hydroxyglutaric aciduria (D-2-HGA), l-2-hydroxyglutaric aciduria (L-2-HGA), and combined d,l-2-hydroxyglutaric aciduria (D,L-2-HGA) cause neurological impairment at young age. Accumulation of d-2-hydroxyglutarate (D-2-HG) and/or l-2-hydroxyglutarate (L-2-HG) in body fluids are the biochemical hallmarks of these disorders. The current review describes the knowledge gathered on 2-hydroxyglutaric acidurias (2-HGA), since the description of the first patients in 1980. We report on the clinical, genetic, enzymatic and metabolic characterization of D-2-HGA type I, D-2-HGA type II, L-2-HGA and D,L-2-HGA, whereas for D-2-HGA type I and type II novel clinical information is presented which was derived from questionnaires.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gregersen et al (1977) were the first to identify enantiomeric d- and l-2-hydroxyglutaric acids (D-2-HG and L-2-HG) as normal constituents of human urine. Three years later, two novel inborn errors of metabolism were simultaneously reported in the Journal of Inherited Metabolic Disease. Chalmers et al (1980) identified a patient with d-2-hydroxyglutaric aciduria (D-2-HGA), while Duran et al (1980) described a case of l-2-hydroxyglutaric aciduria (L-2-HGA), landmark publications that identified the metabolic hallmarks (D- and L-2-HG) in these disorders. Muntau et al (2000) described a third biochemical variant of 2-hydroxyglutaric aciduria (2-HGA) when they reported three patients with elevated urinary D- and L-2-HG, denoted by these authors as “combined d,l-2-hydroxyglutaric aciduria” (D,L-2-HGA). Major milestones in research on these disorders came with gene discovery: D2HGDH encoding d-2-hydroxyglutarate dehydrogenase (D-2-HGDH)(Achouri et al 2004) and L2HGDH encoding l-2-hydroxyglutarate dehydrogenase (L-2-HGDH)(Rzem et al 2004; Topcu et al 2004). In many D-2-HGA, and the majority of L-2-HGA patients, genetic characterization revealed pathogenic mutations in these genes (Struys et al 2005b; Steenweg et al 2010). Nonetheless, in fully one-half of D-2-HGA patients no mutations in D2HGDH were detected (Kranendijk et al 2010a). Subsequently, we described gain-of-function mutations in isocitrate dehydrogenase 2 (IDH2) which proved causative for the D-2-HG accumulation in previously unclassified D-2-HGA patients (Kranendijk et al 2010b). In sum, the preceding decade has provided tremendous advances in our understanding of the inborn 2-hydroxyglutaric acidurias, which will undoubtedly provide a solid foundation from which to develop novel and effective treatment strategies.
This Review evaluates metabolic, enzymatic, genetic and clinical progress in our understanding of the rare inborn organic acidurias D-2-HGA, L-2-HGA and D,L-2-HGA. Future research and therapeutic perspectives are also briefly discussed.
Enantiomeric d,l-2-hydroxyglutaric acid and its origin
The five-carbon dicarboxylic acid 2-hydroxyglutaric acid (2-HG) possesses a hydroxyl group at the second carbon (Fig. 1) which yields a chiral center. Accordingly, two three-dimensional (3D) structures exist, including D-2-HG and L-2-HG, which represent “non-superimposable” mirror images (Fig. 2). Systemic names are (R)-2-hydroxypentanedioic acid and (S)-2-hydroxypentanedioic acid, respectively, for D- and L-2-HG. Whereas enantiomers share identical chemical and physical properties (melting point, mass, solubility and pKa), their differing 3D-structures result in considerable differences in enzymatic and molecular properties.
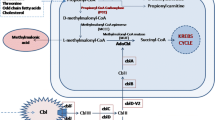
Pilot studies employing stable isotope labeled [13C6]glucose or [2H5]glutamic acid with D-2-HGA lymphoblast cell cultures revealed that mitochondrial 2-ketoglutarate (2-KG), a tricarboxylic acid (TCA) cycle intermediate, can be metabolized to D-2-HG (Struys et al 2004b). Subsequent studies documented the existence of hydroxyacid-oxoacid transhydrogenase (HOT) activity in human liver and fibroblasts, representing the first demonstration of a human enzyme whose catalytic function was production of D-2-HG (Struys et al 2005c). HOT catalyzes the conversion of γ-hydroxybutyrate (GHB) to succinic semialdehyde (SSA) with a stoichiometric production of D-2-HG from 2-KG (Fig. 3). Similarly, pilot studies of L-2-HGA lymphoblasts incubated with [13C6]glucose and [2H5]glutamic acid further delineated that mitochondrial 2-KG is the precursor of L-2-HG (Struys et al 2007). Currently, the only enzyme known to generate L-2-HG from 2-KG in human is l-malate dehydrogenase (L-malDH) (Fig. 3), whose primary catalytic function is the interconversion of l-malate to oxaloacetate (Rzem et al 2007).
The diagnosis of 2-hydroxyglutaric aciduria
The differential diagnosis of 2-hydroxyglutaric aciduria begins with the clinical evaluation of a patient with unexplained developmental delay and/or other neurological dysfunction of unknown etiology, raising suspicion for a metabolic disorder. Provisional diagnosis of the disorder is occasionally suggested by abnormal brain MRI findings. Urinary organic acid screening with gas chromatography-mass spectrometry (GC-MS), performed in multiple metabolic centers, can reveal increased 2-HG, but the chiral configuration remains to be determined. Although the clinical presentation often can suggest either D-2-HGA or L-2-HGA, chiral differentiation performed with GC-MS or liquid chromatography-tandem mass spectrometry (LC-MS/MS) is mandatory for the correct differential diagnosis (Gibson et al 1993a; Struys et al 2004a). Additionally, amino acid analysis in plasma and/or cerebrospinal fluid (CSF) may identify elevated lysine in L-2-HGA. Subsequently, enzymatic and genetic characterization can confirm L-2-HGA, as well as differentiate between the type I or II form of D-2-HGA, representing important information for genetic counseling and future prenatal diagnosis (Steenweg et al 2010; Kranendijk et al 2010a, b). While elevated L-2-HG levels are specific for L-2-HGA and are also detected in D,L-2-HGA, D-2-HG can be elevated in a number of other disorders in addition to D-2-HGA type I and type II, discussed in a separate section of this Review. Some authors have suggested that a potential false positive diagnosis of D- or L-2-HGA may occur with improperly preserved urine samples linked either to nonenzymatic conversion of 2-KG to D/L-2-HG, or via excretion of D/L-2-HG from bacterial or fungal growth in the urine specimen (Kumps et al 2002). We have not, however, experienced this occurrence in our laboratory.
d-2-hydroxyglutaric aciduria type I and II (MIM# 600721 and MIM# 613657)
Recent studies have documented the presence of two groups, D-2-HGA types I and II, of roughly equal size and encompassing >95% of all patients. D-2-HGA type I associates with mutations in the D2HGDH gene encoding d-2-hydroxyglutarate dehydrogenase (D-2-HGDH) which lead to impaired enzyme function (Kranendijk et al 2010a). The type II disorder derives from specific gain-of-function mutations in the IDH2 gene which result in accumulation of D-2-HG (Kranendijk et al 2010b). Both gene defects lead to supraphysiological accumulation of D-2-HG in urine, plasma and CSF, representing the biochemical hallmarks of the diseases.
Clinical manifestations of D-2-HGA
D-2-HGA was recognized as a distinct neurometabolic disorder with mild and severe phenotypes in the late 1990s (Van der Knaap et al 1999a, b). The clinical phenotype encompassed epilepsy, hypotonia and psychomotor retardation as the primary features. With the genetic identification of type I (D2HGDH mutations, MIM# 600721) and type II (gain-of-function mutations in IDH2, MIM# 613657) disorders in 2010, it became clear that studies from 1999 encompassed heterogeneous groups of both subtypes of patients, as well as combined D,L-2-HGA patients, which likely led to the heterogeneous description of mild and severe phenotypes.
Herein we present novel clinical features in 14 type I and 19 type II patients based on clinical questionnaires. The age of onset for type I patients is generally within the first six years, whereas for type II the age of onset was within two years (Table 1). Cardinal clinical manifestations for both disorders include developmental delay, hypotonia and seizures, although seizures occur with higher frequency in type II patients (Table 1). All type II patients were developmentally delayed, and the delays were more severe than those observed in type I patients. In 9 of 19 type II patients cardiomyopathy (primarily dilated, but in one case hypertrophic) was observed, a feature absent from the type I phenotype. The course of the type II disease is primarily progressive, but a static disease, or even improvement have been reported in a limited number. Life expectancies for type II patients may range from several months to early adulthood (Table 1). Conversely, for type I patients the life expectancy remains undefined. One patient died in the third week of life with a diagnosis of necrotizing enterocolitis at post mortem examination, while three others are currently 5-12 years of age.
The disorders appear panethnic, and consanguinity is frequent in the type I disorder while essentially absent in type II families. Clinical heterogeneity of previously published cases showed alikeness with the symptoms summarized in Table 1. Additional clinical features variably reported have included macrocephaly, dysmorphic features and cerebral visual failure. These patients were not, however, differentiated as D-2-HGA type I or type II disease. Cardiomyopathy was exclusive to type II patients in our data (Table 1), however in one reported unrelated type I patient (homozygous for c.458T>C; p.Met153Thr in D2HGDH) an increased cardiothoracic index and hypertrophic cardiomyopathy was documented (Haliloglu et al 2009), which is until now an isolated finding.
Neuroimaging performed in D-2-HGA patients was predominantly instituted prior to knowledge of the underlying molecular defects. The clinical features in these undifferentiated patients included enlargement of the lateral ventricles, enlarged frontal subarachnoid spaces, subdural effusions, subependymal pseudocysts, signs of delayed cerebral maturation and multifocal cerebral white-matter abnormalities (Van der Knaap et al 1999a, b). An ongoing imaging study is underway in our laboratories to more accurately define the CNS abnormalities associated with the type I and II disorders. Finally, an intriguing study of 4.5-year-old female monozygotic twins affected with type I disease (Misra et al 2005) revealed one with multiple congenital anomalies, severe developmental delay, and abnormal neuroradiological findings, while the other reached all major motor and language milestones appropriately associated with a generally uncomplicated clinical course. The latter imply that postzygotic genetic alterations and/or environmental factors influence the phenotypic outcome of the type I disease.
D2HGDH mutations: the molecular basis of D-2-HGA type I
Employing DEAE-sepharose chromatography combined with spectrophotometric and radioactive detection, Achouri and co-workers isolated enzymes from rat liver in order to screen for a dehydrogenase acting upon D-2-HG (Achouri et al 2004). Van Schaftingen and coworkers subsequently identified the D2HGDH gene (GeneBank 728294, NM_152783, MIM# 609186), which encodes a d-2-hydroxyglutarate dehydrogenase (D-2-HGDH) with considerable specificity for D-2-HG. Subsequent detection of D2HGDH mutations in patients with D-2-HGA confirmed the identity of this cDNA and documented the genetic lesions (Misra et al 2005; Struys et al 2005a, b; Haliloglu et al 2009; Kranendijk et al 2010a; Pervaiz et al 2011). Twenty six patients have thus far been identified, harboring 31 mutations spread across the D2HGDH coding sequence (Fig. 4). Eleven mutations are predicted to result in truncated enzymes, while the remaining twenty missense mutations alter amino acids conserved across species, which suggests pathogenicity. As well, limited overexpression studies of mutated alleles have confirmed their pathogenicity (Struys et al 2005b). Accordingly, patients harboring pathogenic homozygous, or compound heterozygous mutations, in the D2HGDH gene are denoted D-2-HGA type I (∼50% of the D-2-HGA population), and the autosomal-recessive inheritance pattern has been confirmed by genetic characterization of parental DNA samples.
IDH2 mutations: the molecular basis of D-2-HGA type II
Various investigators working in the field of cancer genetics identified an increased incidence of heterozygous mutations in isocitrate dehydrogenase 1 and 2 (IDH1, IDH2) in selected individuals with carcinoma. These alleles induced novel enzyme functions in IDH1 and IDH2, namely the newfound capacity to convert 2-KG to D-2-HG, and resulting in supraphysiological accumulation of D-2-HG (Parsons et al 2008; Dang et al 2009; Ward et al 2010). These data led our group to hypothesize that IDH mutations might represent the genetic underpinning of D-2-HGA, and we subsequently identified germline heterozygous IDH2 mutations in a number of D-2-HGA patients (GeneBank 3418, NM_002168, MIM# 147650)(Kranendijk et al 2010b). These novel molecular events thereby represent the second D-2-HGA defect, denoted as D-2-HGA type II. We have subsequently identified 14 patients carrying the heterozygous c.419G>A, p.Arg140Gln mutation (IDH2wt/R140Q), in addition to one patient who was heterozygous for c.418C>G, p.Arg140Gly (IDH2wt/R140G). In the DNA derived from 24 patients, these gain-of-function mutations represent ∼50% of the D-2-HGA population. Moreover, IDH2 alleles were not detected in 8 of 9 sets of parents, suggesting de novo occurrence and an autosomal dominant pattern of inheritance. Of interest, in one family three subsequent pregnancies were diagnosed as affected by D-2-HGA type II (genetic diagnosis in DNA isolated from amniocytes), which suggested germline mosaicism in the mother who was subsequently confirmed to harbor somatic mosaicism in blood.
Currently, 95 D-2-HGA patients have been detected (including unpublished patients identified in our laboratories), most of whom were diagnosed prior to identification of the molecular lesions (Table 2). For ∼50% of those patients, genetic characterization revealed 26 type I and 24 type II patients. In two cases no mutations were found in either gene (D2HGDH or IDH2), and thus the underlying etiology remains unknown. Several patients have previously been reported as D-2-HGA who showed (to lesser extent) increased L-2-HG as well, denoted “combined D,L-2-HGA”, which will be discussed later in detail. Another subset of D-2-HGA patients, without D2HGDH or IDH2 mutations, but with skeletal dysplasia is discussed more completely later in this Review.
Metabolism in D-2-HGA
Different catalytic mechanisms underlie D-2-HG accumulation in the D-2-HGA type I and II disorders. HOT (EC 1.1.99.24) converts 2-KG to D-2-HG (Fig. 5)(Struys et al 2005c), and currently there is no known physiological function for D-2-HG in human metabolism. To maintain carbon balance and avoid intoxication, D-2-HG is interconverted to 2-KG via D-2-HGDH (EC 1.1.99.-). Our laboratory demonstrated impaired D-2-HGDH activity in lymphoblasts and fibroblasts derived from type I patients, whereas this activity was normal in type II cells (Table 3) (Wickenhagen et al 2009; Kranendijk et al 2010a). Thus, D-2-HG accumulation in the type I disease is directly correlated with deficient D-2-HGDH activity.
In the type II disorder, heterozygous mutations detected in isocitrate dehydrogenase 2 (IDH2, EC 1.1.1.42) at residue 140 replaces arginine by glutamine or glycine (Kranendijk et al 2010b). While wild type IDH2 reversibly catalyzes the conversion of isocitrate to 2-KG using the NADP(H) couple, IDH2wt/R140Q-mutant acquires the enzymatic capacity to convert 2-KG to D-2-HG using NADPH as a hydride donor (Fig. 6). Along these lines, an eight-fold increase of IDH2wt/R140Q reaction velocity was detected in lymphoblasts obtained from type II patients in comparison to control and type I cells (Table 3) (Kranendijk et al 2011). We have speculated that the capacity of active D-2-HGDH is insufficient to metabolize the excess D-2-HG formed by gain-of-function IDH2wt/R140Q.
Different mechanisms leading to the production of D-2-HG in the type I and II disorders appear to be reflected in the absolute concentrations of this metabolite in physiological fluids and intracellularly in cultured lymphoblasts (Table 4) (Kranendijk et al 2010a, b; Kranendijk et al 2011). D-2-HG levels are 2 to 8-fold higher in type II as opposed to type I patients, although the absolute level of D-2-HG is highly increased in all fluids, or cells, that were examined. Metabolite concentrations in plasma exceed those in CSF for both groups. Moreover, extensive metabolic screening (e.g., organic acids, amino acids, acylcarnitines, GABA) has been performed in multiple physiological fluid samples derived from patients, yet the only consistent biochemical marker remains D-2-HG in both subtypes, with normal levels of L-2-HG in all instances.
Pathophysiology of D-2-HGA
Although genetically distinct, D-2-HGA types I and II share accumulation of D-2-HG in physiological fluids, as well as common clinical features, but without cardiomyopathy in type I. These observations suggest that the metabolite D-2-HG contributes to the pathophysiology associated with the clinical features of developmental delay, hypotonia and seizures observed in both groups. Overproduction of D-2-HG likely begins in the mitochondria, since D-2-HGDH and IDH2 are mitochondrial enzymes. Intracellular/mitochondrial D-2-HG concentrations are unknown, but plasma concentrations are often used as a surrogate measure. Plasma D-2-HG concentrations are 30-840 fold increased in patients (26-757 μM)(Table 4). The mean plasma levels are ∼5 times higher in type II than in type I patients with little overlap of the ranges. Since the frequency and severity of developmental delay, hypotonia and seizures observed in type II patients are slightly higher than those observed in the type I disorder (Table 1), there appears to be a correlation between increasing D-2-HG concentrations and disease severity.
In vitro studies have revealed both cyto- and neurotoxic effects of high levels of D-2-HG. Along these lines, exposure of tissues from rat and chick to increasing D-2-HG concentrations downregulated the creatine kinase, complex IV and complex V enzymes (da Silva et al 2002; Kölker et al 2002; da Silva et al 2003a, b, 2004). Additionally, high levels of D-2-HG induced oxidative stress and markedly impaired mitochondrial energy metabolism in several model systems in vitro (Kölker et al 2002; Latini et al 2003b, 2005). Neurotoxic effects of increased D-2-HG was revealed by increased synaptosomal glutamate uptake without alteration in other synaptosomal parameters studied (Junqueira et al 2004), as well as by NMDA receptor activation in primary neuronal cultures (Kölker et al 2002).
The IDH-mutated gain-of-function allele received considerable attention in the broad areas of cancer research, since it was first identified in brain tumors (Parsons et al 2008). Of interest, neoplastic disorders have not been reported in either D-2-HGA type I or II patients, which would argue against the hypothesis proposed by others that D-2-HG is an onco-metabolite (Dang et al 2009; Ward et al 2010). On the other hand, Zhao et al (2009) demonstrated that the hypoxia-inducible factor subunit-1α (HIF-1α), a transcription factor that facilitates tumor growth in the presence of low oxygen, was higher in human gliomas harboring an IDH1 mutation than tumors lacking a mutation. Xu et al (2011) reported that D-2-HG acts as a competitive inhibitor of multiple 2-KG-dependent dioxygenases which leads to a genome-wide alteration in histone and DNA methylation patterns. These authors suggested that the latter may hypothetically contribute to tumorigenesis through alterations of epigenetic control and changes in stem cell differentiation. The preceding observations, focused on epigenetic effects and outcomes, could be in line with our findings in monozygotic twins affected with D-2-HGA type I (Misra et al 2005). Random affects of gene methylation combined with environmental influences during pregnancy and/or after birth might influence phenotypic outcomes, although the twins are genetically identical.
Therapeutic perspectives
There is currently no effective therapeutic intervention for D-2-HGA. Addition of FAD to purified D-2-HGDH enzymes did not result in increased activity (Achouri et al 2004). A therapeutic trial with riboflavin has not been attempted, but might be beneficial since the DNA sequence of D2HGDH belongs to a family of enzymes that use FAD as a cofactor. In the type II disease, inhibition of IDH2-mutants with oxaloacetate led to decreased D-2-HG production in patient lymphoblast lysates (Kranendijk et al 2011). Inhibition with highly IDH2-mutant specific substrates/drugs may represent a viable therapeutic strategy in patients.
l-2-hydroxyglutaric aciduria (MIM# 236792)
l-2-hydroxyglutaric aciduria (L-2-HGA) is an autosomal recessive encephalopathy. It is a “disorder of metabolite repair” (Van Schaftingen et al 2008), but the pathophysiology of the white matter abnormalities is poorly understood.
Clinical manifestations of L-2-HGA
Several clinical reports of L-2-HGA have appeared since the index case in 1980 (Barth et al 1993; Barbot et al 1997; Achouri et al 2004; Topcu et al 2005; Steenweg et al 2009, 2010). The phenotype is homogeneous (Table 5). Steenweg and co-workers described the largest cohort of 106 patients. An insidious onset of disease starting in childhood, developmental delay, epilepsy and cerebellar ataxia were the cardinal clinical signs (Steenweg et al 2010). Accordingly, the course of the disease is slowly progressive. Therefore, mild L-2-HGA patients often remain undiagnosed until adolescence or even adulthood. Virtually all patients display delayed mental and motor development, and about two-third of them epilepsy and cerebellar dysfunction. In about half of patients, macrocephaly and extrapyramidal symptoms including tremor and dystonia are observed. Hypotonia was most prevalent in the earlier stages and spasticity in the latter stages of disease in their cohort. Neurological decompensation (e.g., loss of milestones, such as unassisted walking and the development of speech deficits) was also present in a quarter of the patients.
Steenweg et al (2009) systematically evaluated the brain MRIs of 56 patients, and found a highly characteristic pattern of MRI abnormalities in L-2-HGA including abnormalities of the subcortical cerebral white matter dentate nucleus, globus pallidus, putamen, and caudate nucleus. These results correlated well with previous case reports of L-2-HGA patients (Barth et al 1993; Barbot et al 1997; Topcu et al 2005). As the disease progresses, abnormalities of white matter and basal ganglia signal intensities became more diffuse, followed by cerebral white matter atrophy. Barbot et al (1997) found a strong correlation between the severity of clinical manifestations and the extent of the MRI lesions in a cohort of six patients, but this correlation was not noted by Steenweg et al (2009).
Acute metabolic derangement has not been reported in L-2-HGA, although rapid deterioration has been noted in nine cases, in relation to brain tumors (Moroni et al 2004; Haliloglu et al 2008; Aghili et al 2009). Additionally, Vilarinho et al (2005) reported two children and one adult with brain astrocytomas in their series of 21 L-2-HGA patients of Portuguese descent, while Steenweg et al (2009) mentioned only a single case affected with a tumor in the cerebral cortex in their cohort of 56. The preceding 13 cases of patients with brain tumors suggest an association of L-2-HGA with CNS tumors, which has led to speculation that L-2-HG may predispose to oncogenesis. Non-CNS tumors have been documented in L-2-HGA as well, including a bone tumor involving the right frontal region of the calvaria in one patient (Larnaout et al 2007) and nephroblastoma (Wilms tumor) in a second patient (Rogers et al 2010). On the other hand, a subsequent study in 21 Wilms tumor tissues did not reveal increased L-2-HG levels, which therefore did not directly relate L-2-HG with Wilms tumor formation (Rakheja et al 2011). The possible association of the metabolite L-2-HG and tumorigenesis requires further investigation.
L2HGDH mutations: the molecular basis of L-2-HGA
Two research groups independently identified the gene associated with L-2-HGA. Rzem et al (2004) purified a dehydrogenase from rat liver that catalyzed the conversion of L-2-HG to 2-KG. Various assay methodologies identified several properties of the proposed l-2-hydroxyglutarate dehydrogenase (L-2-HGDH) which facilitated database searches and an eventual identification of the L2HGDH gene (GeneBank 79944, NM_024884, MIM# 609584). Subsequently, homozygosity mapping in three unrelated consanguineous families with L-2-HGA confirmed the association between the L2HGDH gene and l-2-hydroxyglutaric aciduria. An additional homozygosity mapping study identified C14orf160, a gene carrying mutations in 21 confirmed L-2-HGA patients from 15 Turkish families (Topcu et al 2004).
Numerous mutations in L2HGDH have now been reported worldwide, summarized in a Mutation Update (Steenweg et al 2010) and registered in the Leiden Open Variation Database (www.LOVD.nl/L2HGDH 2011). Currently, 86 unique variants have been described in 164 individuals who are homozygous or compound heterozygous (157 index, seven siblings) for these alleles. The majority of variants are missense mutations that alter invariably conserved amino acids.
Metabolism in L-2-HGA
L-2-HG is formed from 2-KG in a non-specific reaction catalyzed by l-malate dehydrogenase (L-malDH, EC 1.1.1.37), which employs NADH as the hydride donor (Fig. 7) (Rzem et al 2007). This reaction is hypothesized to be an “unwanted” side reaction of L-malDH, which normally catalyzes the conversion of l-malate to oxaloacetate in the TCA cycle. Accordingly, to prevent loss of carbon moieties in the TCA cycle, and to protect against potential toxic effects, L-2-HG is reconverted to 2-KG via L-2-HGDH (EC 1.1.99.2), with FAD as cofactor. The role of L-2-HGDH, therefore, appears to be that of a “repair” mechanism for this unwanted side reaction of L-malDH, since L-2-HG has no known physiological function in human. L-2-HGA can thus be considered a “disorder of metabolite repair” (Van Schaftingen et al 2008).
The biochemical hallmark of L-2-HGA is the accumulation of urinary L-2-HG, which is 10 to 300 times increased in comparison to controls (Table 6), but correlation of excretion levels with disease severity have not been demonstrated (Barbot et al 1997; Steenweg et al 2010). The relationship between molecular variants and biochemical abnormalities (increased L-2-HG) in L-2-HGA was confirmed with a specific L-2-HGDH enzyme assay employing stable isotope labeled l-[3,3,4,4-2H4]2-hydroxyglutaric acid and LC-MS/MS (Kranendijk et al 2009). Impaired L-2-HGDH enzyme activity was documented in fibroblasts, lymphoblasts and lymphocytes derived from 15/15 L-2-HGA patients, and in only one residual activity was detected (20% of control). Of interest, this patient was compound heterozygous for two missense mutations, which is considered a relatively mild genotype. On the other hand, some interesting associations have been observed between L2HGDH variants and urinary L-2-HG concentrations. Patients harboring two missense mutations excreted 25-50% less L-2-HG than those with two presumed null mutations (Table 6), suggesting residual enzyme activity in the former group (Steenweg et al 2010). Transfection studies in human embryonic kidney cells overexpressing human L2HGDH have confirmed the absence of L-2-HGDH enzyme activity when pathogenic mutations were incorporated (Rzem et al 2006). L-2-HG levels in plasma and CSF are elevated as well, with a CSF/plasma ratio >1 (Table 7). This is contrarily to the findings in D-2-HGA where D-2-HG concentrations in CSF are lower than in plasma (ratio CSF/plasma <1, Table 4).
In addition to L-2-HGA, lysine is also moderately elevated in CSF and plasma derived from patients (Table 7). Increased urinary levels of lysine were identified in 4 out of 6 patients (Barbot et al 1997), but Barth et al (1993) failed to demonstrate this abnormality in the cohort those investigators studied. Direct correlations between L-2-HG and lysine levels in any physiological fluid have not been documented. Kamoun et al (2002) noted that hyperlysinemia (MIM #238700) is observed secondarily in disorders in which 2-KG is decreased. Since the ε-amino group of lysine is transaminated to 2-KG through the intermediate saccharopine to form 2-aminoadipic semialdehyde and glutamic acid, representing a two step mitochondrial process involving alpha-aminoadipic semialdehyde synthase (AASS), these authors have suggested that lysine accumulation in L-2-HGA reflects low mitochondrial 2-KG availability.
Pathophysiology of L-2-HGA
Exposure of rat brain tissues to increased L-2-HG significantly inhibited creatine kinase activity in rat cerebellum homogenates (da Silva et al 2003c), induced oxidative stress (Latini et al 2003a) and increased glutamate uptake in synaptosomes and synaptic vesicles (Junqueira et al 2003), indicating a potential neurodegenerative effect of L-2-HG. Similar results were previously observed with high levels of D-2-HG, so this outcome is not specific for L-2-HG. Conversely, the characteristic pattern of brain MRI abnormalities in L-2-HGA supports the hypothesis that a specific pathophysiological mechanism exists in L-2-HGA, probably L-2-HG concentration-dependent, but this remains to be conclusively demonstrated. The role of lysine accumulation in the disease pathology remains uncertain, since hyperlysinemia is observed in impaired AASS enzyme activity, a rare metabolic disorder considered to be a “nondisease”, since patients generally achieve normal intellectual performance (Dancis et al 1983; Saudubray and Rabier 2007).
Therapeutic approaches
Specific therapeutic approaches in L-2-HGA have not been reported, although two anecdotal reports have provided provocative results and potential avenues for exploration. L-2-HGA was documented in a 40-year-old female with mild limb spasticity, marked dystonia of the neck and arms, mild intellectual delay, and a homozygous mutation in L2HGDH (Samuraki et al 2008). Brain MRI revealed diffuse atrophy and leukoencephalopathy involving mainly subcortical white matter. Intervention with FAD (30 mg/day) and levocarnitine chloride (900 mg/day) was instituted, resulting in gradual improvement in tremor and dystonia within weeks. After six months of intervention, gait was normal and urinary L-2-HG had decreased by 50%, and this improvement was maintained for more than 4 years following initiation of treatment.
The second patient was a 16-year-old boy with L-2-HGA who presented developmental delay since infancy, but alterations in L2HGDH were not reported. He was unable to walk without support until 3 years of age (Yilmaz 2009), associated with impaired language skills and difficulties with hand movements. Cognitive function became increasingly impaired with age. Intervention with riboflavin (vitamin B2, precursor of FAD; 100 mg/day) partially improved cognitive and motor performance within days, and the urinary L-2-HG level decreased by 75% within three months of treatment. Cessation of riboflavin treatment resulted in significant decompensation (including clinical symptoms and urinary L-2-HG excretion). Reinstitution of riboflavin intervention returned the patient to his previously improved clinical picture within days. His brain MRI showed leukodystrophic changes prior to therapy, which remained unchanged after two years of riboflavin intervention.
In contrast to these two patients, a two month therapeutical trial with riboflavin (200 mg/day) was unsuccessful in a 9-year-old female with L-2-HGA (Jequier et al 2008). She developed a progressive action tremor, light gait ataxia, dysarthria and moderate mental retardation associated with a homozygous splice site mutation in L2HGDH. L-2-HG levels in physiological fluids remained unchanged, suggesting that riboflavin was unable to augment a complete absence of residual enzyme activity associated with the splicing variant.
The fact that L-2-HGDH employs FAD as co-substrate (Fig. 7) and that its enzyme increases with increasing FAD concentrations (Rzem et al 2006), suggest that FAD acts in a chaperone function to restore L-2-HGDH enzyme activity and thereby reduces L-2-HGA excretion. Nevertheless, it appears that this approach (FAD supplementation) is only effective in “mild” missense mutations in L2HGDH as found in the first patient, whereas truncated enzymes (presumed null mutations) are not responsive (Table 6). One patient, having compound heterozygous missense mutations in L2HGDH, was found to have modest residual L-2-HGDH enzyme activity (Kranendijk et al 2009), but a therapeutic trial with riboflavin has not been undertaken. Unfortunately, riboflavin intervention is not expected to improve the leukoencephalopathy observed in patients.
Combined d,l-2-hydroxyglutaric aciduria
Currently, six cases of d,l-2-hydroxyglutaric aciduria (D,L-2-HGA) have been described, of which four were previously classified as D-2-HGA affected with a severe phenotype (Wagner et al 1998; Amiel et al 1999; Van der Knaap et al 1999a, b; Muntau et al 2000; Wajner et al 2002; Read et al 2005). D,L-2-HGA is biochemically characterized by moderately increased D-2-HG and L-2-HG in urine, mild increases of both metabolites in plasma, and a very slight elevation of D-2-HG in CSF and normal levels of L-2-HG (Table 8). D-2-HG concentration exceeds L-2-HG in all fluids, while lactate and TCA cycle metabolites (succinate, fumarate and malate) were variably increased in urine, and 2-ketoglutarate was consistently increased in four patients (A-D).
Five patients (A-D, F) manifested a consistent clinical picture encompassing severe neonatal epileptic encephalopathy, often accompanied by respiratory insufficiency requiring artificial ventilation (Table 9). Four probands died within the first year, and the fifth at 3.5 years. The brain MRI of all revealed enlarged ventricles, subependymal pseudocysts and delayed gyration and myelination. Patient E additionally manifested hypotonia, developmental delay, seizures, cardiomyopathy and respiratory distress, with a brain MRI suggestive of mitochondrial disease (Wajner et al 2002). The patient expired at 10 months of age in cardiogenic shock. The clinical features of patient E show similarities with D-2-HGA type II, although the increased L-2-HG and brain MRI abnormalities deviate with those of D-2-HGA type II. Therefore we speculate that the etiology in this patient is different from that of the other patients.
We speculate that increased 2-KG in patients with D,L-2-HGA lead to the formation of D-2-HG and L-2-HG via HOT and L-malDH activities (Fig. 3). Nonetheless, the D,L-2-HGA disorder appears distinct from D-2-HGA and L-2-HGA, with respect to the much lower level of both isomers in physiological fluids, as well as the presence of increased lactate and TCA cycle intermediates (2-KG, succinate, fumarate and malate), suggestive of mitochondrial dysfunction. No mutations were detected in either D2HGDH, L2HGDH, or IDH (unpublished data) from patients B and F. We speculate that the mode of inheritance in D,L-2-HGA is autosomal recessive since patients C and D were from a consanguineous union. Currently, treatment options are unavailable for combined D,L-2-HGA.
Other disorders with accumulation of 2-HG
Neoplastic disorders with IDH mutations
Mutations in isocitrate dehydrogenase 1 and 2 (IDH1, IDH2), confer on these enzymes a new function, namely the capacity to convert 2-KG to D-2-HG (Parsons et al 2008; Dang et al 2009; Ward et al 2010). This mechanism is identical to that observed in D-2-HGA type II. Consequently, elevated D-2-HG has been observed in samples of malignant gliomas and tumor tissues (Dang et al 2009), as well as in cells and serum obtained from acute myeloid leukemia patients (Ward et al 2010; Gross et al 2010; Sellner et al 2010). All of these pathological conditions showed mutations in either IDH1 or IDH2. Many investigators suggest that D-2-HG is an onco-metabolite, but this has not been proven.
D-2-HGA in skeletal dysplasia (MIM# 271550)
Three patients with skeletal dysplasia manifested increased D-2-HG excretion, a feature not previously reported in either D-2-HGA type I or II. Talkhani et al (2000) reported a patient with spondyloenchondrodysplasia and elevated D-2-HG in urine and plasma, and normal L-2-HG concentration. The proband was diagnosed at 1 year with a crippling form of skeletal dysplasia, and global developmental delay that improved in her second year of life. The second patient was a male infant diagnosed at 6 months with skeletal dysplasia, and mild motor and mental delay (Honey et al 2003). Urinalysis revealed increased D-2-HG. At 11 months the cardinal clinical feature was spondyloenchondrodysplasia associated with hypotonia, delayed fine motor function, and severely delayed speech development. Brain MRI revealed moderate dilatation of the lateral ventricles, a large cavum septi pellucidi and cavum vergae, with normal white matter maturation. The third proband was a 17-month-old boy with waddling gait and swollen joints, whose motor and mental development (other than delayed walking) was normal at 3 years of age (Bayar et al 2005). A bone survey revealed severe metaphyseal widening, splaying, cupping and fragmentation. D-2-HG was elevated in the urine on two occasions. The clinical and metabolic similarities led Bayar and colleagues to label it as “metaphyseal enchondrodysplasia with d-2-hydroxyglutaric aciduria”, since this cohort of patients distinctly differ radiographically and biochemically from other cases of spondyloenchondrodysplasia.
Very recently, Vissers et al (2011) detected in four patients affected with metaphyseal chondromatosis and d-2-hydroxyglutaric aciduria (MC-HGA), including the patient reported by Bayar et al, somatic mutations of IDH1 (p.Arg132His, p.Arg132Ser) using exome sequencing of blood DNA. These somatic mutations in IDH1 may explain all features of MC-HGA, including the sporadic occurrence, metaphyseal disorganization and chondromatosis, urinary excretion of D-2-HG, and reduced cerebral myelinization.
Multiple Acyl-CoA dehydrogenase deficiency (MADD, MIM# 231680, glutaric aciduria type II)
MADD is an autosomal recessively-inherited disorder of fatty acid, amino acid, and choline metabolism (Olsen et al 2007; Liang et al 2009). MADD can result from defects in two flavoproteins: electron transfer flavoprotein (ETF) or ETF:ubiquinone oxidoreductase (ETF:QO). ETF is an electron acceptor for several dehydrogenases, subsequently transferring these to ETF-QO which then moves these electrons to ubiquinone further along in the respiratory chain. In MADD, glutaric aciduria is the biochemical hallmark, the elevated D-2-HG is often observed in urine. The pathophysiology is thought to involve impaired D-2-HGDH activity, since it cannot transfer its electrons to defective ETF or ETF:QO.
Succinic semialdehyde dehydrogenase deficiency (SSADH, MIM# 271980)
SSADH deficiency is an autosomal recessively inherited disorder caused by mutations in the SSADH gene (Jakobs et al 1993; Akaboshi et al 2003). SSADH catalyzes the conversion of succinic semialdehyde (SSA) to succinic acid (SA). SSA accumulates and is converted to 4-hydroxybutyrate (GHB) via SSA-reductase. GHB represents the biochemical hallmark of this disease. The proposed pathophysiology is that elevated intracellular GHB drives the HOT reaction to form SSA and increased D-2-HG in stoichiometric conversion from 2-KG (Fig. 3) (Struys et al 2006).
Miscellaneous disorders related with 2-HG
Other disorders show increased levels of 2-HG (undifferentiated for D-2-HG or L-2-HG, or combined accumulation of both), often related to a primary accumulation of 2-KG. For example, DOOR-syndrome (an autosomal recessive malformation syndrome) variably features elevated urinary 2-KG and 2-HG (James et al 2007). Also dihydrolipoyl dehydrogenase (E3) deficiency manifests increased levels of many organic acids, including 2-KG and 2-HG (Kuhara et al 1983).
Final remarks
The inborn 2-hydroxyglutaric acidurias represent distinct neurometabolic disorders for which the underlying etiology is only beginning to be revealed (summarized in Table 10 and supplemented with a diagnostic flow chart in Fig. 8). Although the D-, L- and combined disorders appear to form distinct clinical diseases, we remain hampered in our understanding of their pathophysiology, because the role of the metabolites themselves remains unclear. Specific metabolic pathways for these chiral intermediates are largely unknown, and their role in the central nervous system remains to be defined. The development of models (yeast, zebrafish, mouse) in which the specific genes are ablated/mutated (D2HGDH, L2HGDH, IDH1, IDH2) may help us to understand the associated biochemistry. Nonetheless, the current work has set the foundation for a more comprehensive understanding of these disorders, based upon clinical, metabolic, enzymatic and genetic characterization. As is the case with most “new” neurometabolic disorders, the heterogeneity of phenotypic/genotypic presentations is likely to expand as our knowledge of the disorders grows. Accurate working models combined with low-threshold screening can provide clues to therapeutic strategies.
Abbreviations
- 2-HG:
-
2-hydroxyglutaric acid (undifferentiated for chiral d- or l-form)
- 2-HGA:
-
2-hydroxyglutaric aciduria
- D-2-HG:
-
d-2-hydroxyglutaric acid
- D-2-HGA:
-
d-2-hydroxyglutaric aciduria
- D-2-HGDH:
-
d-2-hydroxyglutarate dehydrogenase enzyme
- D2HGDH :
-
gene encoding d-2-hydroxyglutarate dehydrogenase
- L-2-HG:
-
l-2-hydroxyglutaric acid
- L-2-HGA:
-
l-2-hydroxyglutaric aciduria
- L-2-HGDH:
-
l-2-hydroxyglutarate dehydrogenase enzyme
- L2HGDH :
-
gene encoding l-2-hydroxyglutarate dehydrogenase
- D,L-2-HGA:
-
combined d,l-2-hydroxyglutaric aciduria
- IDH2 :
-
gene encoding isocitrate dehydrogenase 2
- 2-KG:
-
2-ketoglutaric acid
- HOT:
-
hydroxyacid-oxoacid transhydrogenase enzyme
- L-malDH:
-
l-malate dehydrogenase enzyme
- MIM:
-
Mendelian Inheritance in Man
- CSF:
-
cerebrospinal fluid
References
Achouri Y, Noel G, Vertommen D, Rider MH, Veiga-da-Cunha M, Van Schaftingen E (2004) Identification of a dehydrogenase acting on D-2-hydroxyglutarate. Biochem J 381:35–42
Aghili M, Zahedi F, Rafiee E (2009) Hydroxyglutaric aciduria and malignant brain tumor: a case report and literature review. J Neurooncol 91:233–236
Akaboshi S, Hogema BM, Novelletto A et al (2003) Mutational spectrum of the succinate semialdehyde dehydrogenase (ALDH5A1) gene and functional analysis of 27 novel disease-causing mutations in patients with SSADH deficiency. Hum Mutat 22:442–450
Amiel J, De Lonlay P, Francannet C et al (1999) Facial anomalies in D-2-hydroxyglutaric aciduria. Am J Med Genet 86:124–129
Baker NS, Sarnat HB, Jack RM, Patterson K, Shaw DW, Herndon SP (1997) D-2-hydroxyglutaric aciduria: hypotonia, cortical blindness, seizures, cardiomyopathy, and cylindrical spirals in skeletal muscle. J Child Neurol 12:31–36
Barbot C, Fineza I, Diogo L et al (1997) L-2-Hydroxyglutaric aciduria: clinical, biochemical and magnetic resonance imaging in six Portuguese pediatric patients. Brain Dev 19:268–273
Barth PG, Hoffmann GF, Jaeken J et al (1992) L-2-hydroxyglutaric acidemia: a novel inherited neurometabolic disease. Ann Neurol 32:66–71
Barth PG, Hoffmann GF, Jaeken J et al (1993) L-2-hydroxyglutaric acidaemia: clinical and biochemical findings in 12 patients and preliminary report on L-2-hydroxyacid dehydrogenase. J Inherit Metab Dis 16:753–761
Bayar A, Acun C, Dursun A et al (2005) Metaphyseal enchondrodysplasia with 2-hydroxy-glutaric aciduria: observation of a third case and further delineation. Clin Dysmorphol 14:7–11
Chalmers RA, Lawson AM, Watts RW et al (1980) D-2-hydroxyglutaric aciduria: case report and biochemical studies. J Inherit Metab Dis 3:11–15
Clarke NF, Andrews I, Carpenter K, Jakobs C, Van der Knaap MS, Kirk EP (2003) D-2-hydroxyglutaric aciduria: a case with an intermediate phenotype and prenatal diagnosis of two affected fetuses. Am J Med Genet A 120A:523–527
Craigen WJ, Jakobs C, Sekul EA et al (1994) D-2-hydroxyglutaric aciduria in neonate with seizures and CNS dysfunction. Pediatr Neurol 10:49–53
da Silva CG, Ribeiro CA, Leipnitz G et al (2002) Inhibition of cytochrome c oxidase activity in rat cerebral cortex and human skeletal muscle by D-2-hydroxyglutaric acid in vitro. Biochim Biophys Acta 1586:81–91
da Silva CG, Bueno AR, Rosa RB et al (2003a) Inhibition of mitochondrial creatine kinase activity by D-2-hydroxyglutaric acid in cerebellum of young rats. Neurochem Res 28:1329–1337
da Silva CG, Bueno AR, Schuck PF et al (2003b) D-2-hydroxyglutaric acid inhibits creatine kinase activity from cardiac and skeletal muscle of young rats. Eur J Clin Invest 33:840–847
da Silva CG, Bueno AR, Schuck PF et al (2003c) L-2-hydroxyglutaric acid inhibits mitochondrial creatine kinase activity from cerebellum of developing rats. Int J Dev Neurosci 21:217–224
da Silva CG, Bueno AR, Schuck PF et al (2004) Inhibition of creatine kinase activity from rat cerebral cortex by D-2-hydroxyglutaric acid in vitro. Neurochem Int 44:45–52
Dancis J, Hutzler J, Ampola MG et al (1983) The prognosis of hyperlysinemia: an interim report. Am J Hum Genet 35:438–442
Dang L, White DW, Gross S et al (2009) Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature 462:739–744
Duran M, Kamerling JP, Bakker HD, van Gennip AH, Wadman SK (1980) L-2-Hydroxyglutaric aciduria: an inborn error of metabolism? J Inherit Metab Dis 3:109–112
Eeg-Olofsson O, Zhang WW, Olsson Y, Jagell S, Hagenfeldt L (2000) D-2-hydroxyglutaric aciduria with cerebral, vascular, and muscular abnormalities in a 14-year-old boy. J Child Neurol 15:488–492
Geerts Y, Renier WO, Bakkeren J, de Jong J (1996) 2-Hydroxyglutaric aciduria: a case report on an infant with the D-isomeric form with review of the literature. J Neurol Sci 143:166–169
Gibson KM, Ten Brink HJ, Schor DS et al (1993a) Stable-isotope dilution analysis of D- and L-2-hydroxyglutaric acid: application to the detection and prenatal diagnosis of D- and L-2-hydroxyglutaric acidemias. Pediatr Res 34:277–280
Gibson KM, Craigen W, Herman GE, Jakobs C (1993b) D-2-hydroxyglutaric aciduria in a newborn with neurological abnormalities: a new neurometabolic disorder? J Inherit Metab Dis 16:497–500
Gregersen N, Ingerslev J, Rasmussen K (1977) Low molecular weight organic acids in the urine of the newborn. Acta Paediatr Scand 66:85–89
Gross S, Cairns RA, Minden MD et al (2010) Cancer-associated metabolite 2-hydroxyglutarate accumulates in acute myelogenous leukemia with isocitrate dehydrogenase 1 and 2 mutations. J Exp Med 207:339–344
Haliloglu G, Jobard F, Oguz KK et al (2008) L-2-hydroxyglutaric aciduria and brain tumors in children with mutations in the L2HGDH gene: neuroimaging findings. Neuropediatrics 39:119–122
Haliloglu G, Temucin CM, Oguz KK et al (2009) Peripheral neuropathy in a patient with D-2-hydroxyglutaric aciduria. J Inherit Metab Dis Short Report #148
Honey EM, Van Rensburg M, Knoll DP, Mienie LJ, Van de Werke I, Beighton P (2003) Spondyloenchondromatosis with D-2-hydroxyglutaric aciduria: a report of a second patient with this unusual combination. Clin Dysmorphol 12:95–99
Jakobs C, Jaeken J, Gibson KM (1993) Inherited disorders of GABA metabolism. J Inherit Metab Dis 16:704–715
James AW, Miranda SG, Culver K, Hall BD, Golabi M (2007) DOOR syndrome: clinical report, literature review and discussion of natural history. Am J Med Genet A 143A:2821–2831
Jequier GM, Roulet-Perez E, Meagher-Villemure K et al (2008) Sudden unexpected death in an infant with L-2-hydroxyglutaric aciduria. Eur J Pediatr 168:957–962
Junqueira D, Brusque AM, Porciuncula LO et al (2003) Effects of L-2-hydroxyglutaric acid on various parameters of the glutamatergic system in cerebral cortex of rats. Metab Brain Dis 18:233–243
Junqueira D, Brusque AM, Porciuncula LO et al (2004) In vitro effects of D-2-hydroxyglutaric acid on glutamate binding, uptake and release in cerebral cortex of rats. J Neurol Sci 217:189–194
Kamoun P, Richard V, Rabier D, Saudubray JM (2002) Plasma lysine concentration and availability of 2-ketoglutarate in liver mitochondria. J Inherit Metab Dis 25:1–6
Kölker S, Pawlak V, Ahlemeyer B et al (2002) NMDA receptor activation and respiratory chain complex V inhibition contribute to neurodegeneration in d-2-hydroxyglutaric aciduria. Eur J Neurosci 16:21–28
Kranendijk M, Salomons GS, Gibson KM et al (2009) Development and implementation of a novel assay for L-2-hydroxyglutarate dehydrogenase (L-2-HGDH) in cell lysates: L-2-HGDH deficiency in 15 patients with L-2-hydroxyglutaric aciduria. J Inherit Metab Dis 32:713–719
Kranendijk M, Struys EA, Gibson KM et al (2010a) Evidence for genetic heterogeneity in D-2-hydroxyglutaric aciduria. Hum Mutat 31:279–283
Kranendijk M, Struys EA, Van Schaftingen E et al (2010b) IDH2 mutations in patients with D-2-hydroxyglutaric aciduria. Science 330:336
Kranendijk M, Salomons GS, Gibson KM, Van Schaftingen E, Jakobs C, Struys EA (2011) A lymphoblast model for IDH2 gain-of-function activity in D-2-hydroxyglutaric aciduria type II: novel avenues for biochemical and therapeutic studies. Biochim Biophys Acta 1812:1380–1384
Kuhara T, Shinka T, Inoue Y et al (1983) Studies of urinary organic acid profiles of a patient with dihydrolipoyl dehydrogenase deficiency. Clin Chim Acta 133:133–140
Kumps A, Duez P, Mardens Y (2002) Metabolic, nutritional, iatrogenic, and artifactual sources of urinary organic acids: a comprehensive table. Clin Chem 48:708–717
Kwong KL, Mak T, Fong CM, Poon KH, Wong SN, So KT (2002) D-2-Hydroxyglutaric aciduria and subdural haemorrhage. Acta Paediatr 91:716–718
Larnaout A, Amouri R, Neji S, Zouari M, Kaabachi N, Hentati F (2007) Osteoma of the calvaria in L-2-hydroxyglutaric aciduria. J Inherit Metab Dis 30:980
Latini A, Scussiato K, Rosa RB et al (2003a) Induction of oxidative stress by L-2-hydroxyglutaric acid in rat brain. J Neurosci Res 74:103–110
Latini A, Scussiato K, Rosa RB et al (2003b) D-2-hydroxyglutaric acid induces oxidative stress in cerebral cortex of young rats. Eur J Neurosci 17:2017–2022
Latini A, da Silva CG, Ferreira GC et al (2005) Mitochondrial energy metabolism is markedly impaired by D-2-hydroxyglutaric acid in rat tissues. Mol Genet Metab 86:188–199
Liang WC, Ohkuma A, Hayashi YK et al (2009) ETFDH mutations, CoQ10 levels, and respiratory chain activities in patients with riboflavin-responsive multiple acyl-CoA dehydrogenase deficiency. Neuromuscul Disord 19:212–216
Mahfoud A, Dominguez CL, Rashed M et al (2009) D-2-hydroxyglutaric aciduria. Invest Clin 50:369–375
Misra VK, Struys EA, O'Brien W et al (2005) Phenotypic heterogeneity in the presentation of D-2-hydroxyglutaric aciduria in monozygotic twins. Mol Genet Metab 86:200–205
Moroni I, Bugiani M, D'Incerti L et al (2004) L-2-hydroxyglutaric aciduria and brain malignant tumors: a predisposing condition? Neurology 62:1882–1884
Muntau AC, Roschinger W, Merkenschlager A et al (2000) Combined D-2- and L-2-hydroxyglutaric aciduria with neonatal onset encephalopathy: a third biochemical variant of 2-hydroxyglutaric aciduria? Neuropediatrics 31:137–140
Nyhan WL, Shelton GD, Jakobs C et al (1995) D-2-hydroxyglutaric aciduria. J Child Neurol 10:137–142
Olsen RK, Olpin SE, Andresen BS et al (2007) ETFDH mutations as a major cause of riboflavin-responsive multiple acyl-CoA dehydrogenation deficiency. Brain 130:2045–2054
Parsons DW, Jones S, Zhang X et al (2008) An integrated genomic analysis of human glioblastoma multiforme. Science 321:1807–1812
Pervaiz MA, Patterson MC, Struys EA et al (2011) Co-morbidity of Sanfilippo Syndrome type C and D-2-hydroxyglutaric aciduria. J Neurol 258:1564–1565
Rakheja D, Mitui M, Boriack RL, Deberardinis RJ (2011) Isocitrate dehydrogenase 1/2 mutational analyses and 2-hydroxyglutarate measurements in Wilms tumors. Pediatr Blood Cancer 56:379–383
Read MH, Bonamy C, Laloum D et al (2005) Clinical, biochemical, magnetic resonance imaging (MRI) and proton magnetic resonance spectroscopy (1H MRS) findings in a fourth case of combined D- and L-2 hydroxyglutaric aciduria. J Inherit Metab Dis 28:1149–1150
Rogers RE, Deberardinis RJ, Klesse LJ, Boriack RL, Margraf LR, Rakheja D (2010) Wilms tumor in a child with L-2-hydroxyglutaric aciduria. Pediatr Dev Pathol 13:408–411
Rzem R, Veiga-da-Cunha M, Noel G et al (2004) A gene encoding a putative FAD-dependent L-2-hydroxyglutarate dehydrogenase is mutated in L-2-hydroxyglutaric aciduria. Proc Natl Acad Sci U S A 101:16849–16854
Rzem R, Van Schaftingen E, Veiga-da-Cunha M (2006) The gene mutated in L-2-hydroxyglutaric aciduria encodes L-2-hydroxyglutarate dehydrogenase. Biochimie 88:113–116
Rzem R, Vincent MF, Van Schaftingen E, Veiga-da-Cunha M (2007) L-2-hydroxyglutaric aciduria, a defect of metabolite repair. J Inherit Metab Dis 30:681–689
Samuraki M, Komai K, Hasegawa Y et al (2008) A successfully treated adult patient with L-2-hydroxyglutaric aciduria. Neurology 70:1051–1052
Saudubray JM, Rabier D (2007) Biomarkers identified in inborn errors for lysine, arginine, and ornithine. J Nutr 137:1669S–1672S
Sellner L, Capper D, Meyer J et al (2010) Increased levels of 2-hydroxyglutarate in AML patients with IDH1-R132H and IDH2-R140Q mutations. Eur J Haematol 85:457–459
Steenweg ME, Salomons GS, Yapici Z et al (2009) L-2-Hydroxyglutaric aciduria: pattern of MR imaging abnormalities in 56 patients. Radiology 251:856–865
Steenweg ME, Jakobs C, Errami A et al (2010) An overview of L-2-hydroxyglutarate dehydrogenase gene (L2HGDH) variants: a genotype-phenotype study. Hum Mutat 31:380–390
Struys EA, Jansen EE, Verhoeven NM, Jakobs C (2004a) Measurement of urinary D- and L-2-hydroxyglutarate enantiomers by stable-isotope-dilution liquid chromatography-tandem mass spectrometry after derivatization with diacetyl-L-tartaric anhydride. Clin Chem 50:1391–1395
Struys EA, Verhoeven NM, Brunengraber H, Jakobs C (2004b) Investigations by mass isotopomer analysis of the formation of D-2-hydroxyglutarate by cultured lymphoblasts from two patients with D-2-hydroxyglutaric aciduria. FEBS Lett 557:115–120
Struys EA, Korman SH, Salomons GS et al (2005a) Mutations in phenotypically mild D-2-hydroxyglutaric aciduria. Ann Neurol 58:626–630
Struys EA, Salomons GS, Achouri Y et al (2005b) Mutations in the D-2-hydroxyglutarate dehydrogenase gene cause D-2-hydroxyglutaric aciduria. Am J Hum Genet 76:358–360
Struys EA, Verhoeven NM, Ten Brink HJ, Wickenhagen WV, Gibson KM, Jakobs C (2005c) Kinetic characterization of human hydroxyacid-oxoacid transhydrogenase: relevance to D-2-hydroxyglutaric and gamma-hydroxybutyric acidurias. J Inherit Metab Dis 28:921–930
Struys EA, Verhoeven NM, Salomons GS et al (2006) D-2-hydroxyglutaric aciduria in three patients with proven SSADH deficiency: genetic coincidence or a related biochemical epiphenomenon? Mol Genet Metab 88:53–57
Struys EA, Gibson KM, Jakobs C (2007) Novel insights into L-2-hydroxyglutaric aciduria: mass isotopomer studies reveal 2-oxoglutaric acid as the metabolic precursor of L-2-hydroxyglutaric acid. J Inherit Metab Dis 30:690–693
Sugita K, Kakinuma H, Okajima Y, Ogawa A, Watanabe H, Niimi H (1995) Clinical and MRI findings in a case of D-2-hydroxyglutaric aciduria. Brain Dev 17:139–141
Talkhani IS, Saklatvala J, Dwyer J (2000) D-2-hydroxyglutaric aciduria in association with spondyloenchondromatosis. Skeletal Radiol 29:289–292
Topcu M, Jobard F, Halliez S et al (2004) L-2-Hydroxyglutaric aciduria: identification of a mutant gene C14orf160, localized on chromosome 14q22.1. Hum Mol Genet 13:2803–2811
Topcu M, Aydin OF, Yalcinkaya C et al (2005) L-2-hydroxyglutaric aciduria: a report of 29 patients. Turk J Pediatr 47:1–7
Van der Knaap MS, Jakobs C, Hoffmann GF et al (1999a) D-2-hydroxyglutaric aciduria: further clinical delineation. J Inherit Metab Dis 22:404–413
Van der Knaap MS, Jakobs C, Hoffmann GF et al (1999b) D-2-Hydroxyglutaric aciduria: biochemical marker or clinical disease entity? Ann Neurol 45:111–119
Van Schaftingen E, Rzem R, Veiga-da-Cunha M (2008) L-2-Hydroxyglutaric aciduria, a disorder of metabolite repair. J Inherit Metab Dis 32:135–142
Vilarinho L, Cardoso ML, Gaspar P et al (2005) Novel L2HGDH mutations in 21 patients with L-2-hydroxyglutaric aciduria of Portuguese origin. Hum Mutat 26:395–396
Vissers LE, Fano V, Martinelli D et al (2011) Whole-exome sequencing detects somatic mutations of IDH1 in metaphyseal chondromatosis with D-2-hydroxyglutaric aciduria (MC-HGA). Am J Med Genet A 155A:2609–2616
Wagner L, Hoffmann GF, Jakobs C (1998) D-2-hydroxyglutaric aciduria: evidence of clinical and biochemical heterogeneity. J Inherit Metab Dis 21:247–250
Wajner M, Vargas CR, Funayama C et al (2002) D-2-Hydroxyglutaric aciduria in a patient with a severe clinical phenotype and unusual MRI findings. J Inherit Metab Dis 25:28–34
Wang X, Jakobs C, Bawle EV (2003) D-2-Hydroxyglutaric aciduria with absence of corpus callosum and neonatal intracranial haemorrhage. J Inherit Metab Dis 26:92–94
Ward PS, Patel J, Wise DR et al (2010) The common feature of leukemia-associated IDH1 and IDH2 mutations is a neomorphic enzyme activity converting alpha-ketoglutarate to 2-hydroxyglutarate. Cancer Cell 17:225–234
Wickenhagen WV, Salomons GS, Gibson KM, Jakobs C, Struys EA (2009) Measurement of D-2-hydroxyglutarate dehydrogenase activity in cell homogenates derived from D-2-hydroxyglutaric aciduria patients. J Inherit Metab Dis 32:264–268
www.LOVD.nl/L2HGDH (2011) Leiden Open Variation Database - L2HGDH
Xu W, Yang H, Liu Y et al (2011) Oncometabolite 2-Hydroxyglutarate Is a Competitive Inhibitor of alpha-Ketoglutarate-Dependent Dioxygenases. Cancer Cell 19:17–30
Yilmaz K (2009) Riboflavin treatment in a case with L-2-hydroxyglutaric aciduria. Eur J Paediatr Neurol 13:57–60
Zhao S, Lin Y, Xu W et al (2009) Glioma-derived mutations in IDH1 dominantly inhibit IDH1 catalytic activity and induce HIF-1alpha. Science 324:261–265
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: K. Michael Gibson
Competing interest: None declared.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Kranendijk, M., Struys, E.A., Salomons, G.S. et al. Progress in understanding 2-hydroxyglutaric acidurias. J Inherit Metab Dis 35, 571–587 (2012). https://doi.org/10.1007/s10545-012-9462-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10545-012-9462-5