Abstract
Cystathionine β-synthase (CBS) deficiency is usually confirmed by assaying the enzyme activity in cultured skin fibroblasts. We investigated whether CBS is present in human plasma and whether determination of its activity in plasma could be used for diagnostic purposes. We developed an assay to measure CBS activity in 20 μL of plasma using a stable isotope substrate - 2,3,3-2H serine. The activity was determined by measurement of the product of enzyme reaction, 3,3-2H-cystathionine, using LC-MS/MS. The median enzyme activity in control plasma samples was 404 nmol/h/L (range 66–1,066; n = 57). In pyridoxine nonresponsive CBS deficient patients, the median plasma activity was 0 nmol/ho/L (range 0–9; n = 26), while in pyridoxine responsive patients the median activity was 16 nmol/hour/L (range 0–358; n = 28); this overlapped with the enzyme activity from control subject. The presence of CBS in human plasma was confirmed by an in silico search of the proteome database, and was further evidenced by the activation of CBS by S-adenosyl-L-methionine and pyridoxal 5′-phosphate, and by configuration of the detected reaction product, 3,3-2H-cystathionine, which was in agreement with the previously observed CBS reaction mechanism. We hypothesize that the CBS enzyme in plasma originates from liver cells, as the plasma CBS activities in patients with elevated liver aminotransferase activities were more than 30-fold increased. In this study, we have demonstrated that CBS is present in human plasma and that its catalytic activity is detectable by LC-MS/MS. CBS assay in human plasma brings new possibilities in the diagnosis of pyridoxine nonresponsive CBS deficiency.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cystathionine β-synthase (CBS; EC 4.2.1.22) (http://www.chem.qmul.ac.uk/iubmb/enzyme/EC4/2/1/22.html) deficiency (http://www.ncbi.nlm.nih.gov/entrez/dispomim.cgi?id=236200) is a well-known genetic disease affecting the first step in the conversion of homocysteine to cysteine and ultimately to inorganic sulfur. Although this disorder was originally considered a severe multisystem disease that affected connective tissue and central nervous and vascular systems (Mudd et al. 1985), recent reports suggest that an unknown proportion of patients with CBS deficiency may suffer from only a mild vascular form of the disease or may be asymptomatic (Gaustadnes et al. 2000; Skovby et al. 2010). Diagnostic hallmarks of this disease are grossly elevated concentrations of plasma total homocysteine combined with decreased plasma concentrations of cysteine and cystathionine (Stabler et al. 1993) with varying elevations of plasma methionine levels. The DNA analysis may be used to confirm the diagnosis only if mutations with known pathogenicity are found at both patient CBS alleles; its utility is limited in other situations.
Demonstration of decreased enzyme activity is a common diagnostic approach in patients suffering from inborn errors of metabolism (IEM) including CBS deficiency. Since the majority of enzymes relevant for IEMs are located intracellularly, the determination of enzymatic activity requires in most cases sampling of patient tissue ranging from simple venepuncture to biopsies of skin or organs, such as the liver. In addition, laborious, time-consuming and technically demanding culturing of tissue such as skin may be required to produce sufficient amounts of cells to express the enzyme of interest. However, these intracellular enzymes can be released in small amounts into plasma reflecting turnover of the respective tissue(s). The amounts of these non-plasma-specific enzymes in body fluids may increase if cell integrity is impaired; clinical chemistry laboratories utilize this phenomenon and routinely analyze activities of liver-, pancreatic-, muscle-, myocardial-, prostatic- and other organ-specific enzymes as biomarkers of diseases (Bhagavan 2001). Determination of enzymes in extracellular fluids is only rarely used for routine diagnostic purposes in IEMs (e.g., biotinidase or total hexosaminidase).
In our study, we hypothesized that CBS may be released into plasma from organs expressing this enzyme and that its activity may be measurable by a sensitive LC-MS/MS assay employing deuterium-labeled substrates. To explore this hypothesis, we studied the CBS catalytic activity of plasma and stimulatory effect of CBS ligands, the presence of the CBS protein by immunological and by an in silico search, and its possible release to plasma from liver and other organs. Finally, we assessed plasma CBS activity in samples from patients with confirmed CBS deficiency to assess the diagnostic utility of this assay.
Materials and methods
Chemicals
All chemicals, if not stated otherwise, were obtained from Sigma–Aldrich (Prague)
Plasma samples
Archived anonymized plasma samples from controls and patients with CBS deficiency were obtained from the authors’ repositories in the Netherlands, UK, Ireland and the Czech Republic. With the exception of 2 individuals, all patients were treated with pyridoxine at the time of sampling, regardless of their pyridoxine responsiveness (for details see Supplementary Table).
Anonymized samples from individuals with presumed liver, pancreatic and kidney disease (i.e., with elevated plasma levels of alanine aminotransferase, α-amylase and creatinine) were obtained as remnant samples after routine biochemical investigations from the Institute of Clinical Biochemistry and Laboratory Diagnostics of the General Faculty Hospital and The First Medical Faculty of Charles University. All experiments performed using human plasma samples were approved by the Ethics Committee of the General University Hospital, Prague.
Apparatus
The LC-MS/MS system consisted of an Agilent 1100 Series LC System (Agilent Technologies, Palo Alto, CA, USA) coupled with API 3200 triple quadrupole mass spectrometer with electron ion source and operated with Analyst software, Vision 1.4 (Applied Biosystems, Foster City, CA, USA).
Sample preparation
To 25 μL of solution containing 200 mmol/L Tris-HCl (pH 8.6), 1 mmol/L pyridoxal 5′-phosphate and 40 mmol/L 2,3,3-2H-labeled serine (Cambridge Isotope Laboratories, USA), 20 μL of plasma or serum was added. The assay was initiated by addition of 5 μL starting solution containing 280 mmol/L homocysteine thiolactone in 100 mmol/L Tris-HCl (pH 8.6), 10 mmol/L DTT, 1 mol/L HCl and 1.225 mol/L NaOH. This starting solution was prepared freshly before sample processing and was preincubated for 5 min at 37°C after alkalinization with NaOH to allow cleavage of the thiolactone ring. For the measurement of the activation of CBS by S-adenosyl-L-methionine, the final assay mixture contained 0.5 mmol/L SAM. The final assay mixture (total volume 50 μL) was incubated at 37°C for 4 h or was stopped immediately if a blank was processed. The reaction was stopped by acidification of the reaction mixture to pH 1-2 with 100 μL of Internal Standard Solution (EZ:faast; Phenomenex, Torrance, USA) containing 3.3 μmol/L of internal standard 3,3,4,4-2H-labeled cystathionine (CDN Isotopes,Quebec, Canada).
LC-MS/MS analysis
The CBS activity was determined by the LC-MS/MS measurement of the product of enzyme reaction, 3,3-2H-cystathionine, using a commercially available kit for amino acid analysis (EZ:faast; Phenomenex). Sample preparation according to the manufacturer’s instruction involved a solid phase extraction step, derivatization with propyl chloroformate, and an extraction into an organic solvent prior to LC-MS/MS analysis.
The LC-MS/MS analysis was performed on proprietary EZ:faast AAA-MS column (250 × 2.0 mm) using LC and MS settings described in EZ:faast user manual.
The retention time of cystathionine isotopes was 15.5 min; total analysis time including regeneration of the column was 25 min. Detection of the analytes was carried out using positive electrospray ionization technique and selected multiple reaction monitoring. The precursor → product transitions for 3,3-2H-cystathionine (enzyme reaction product; m/z 481.3 → 230.3), cystathionine (calibration standards; m/z 479.3 → 230.3) and 3,3,4,4-2H-labeled cystathionine (internal standard; m/z 483.3 → 234.3) were monitored.
Standards, calibration curves and activity calculation
Quantitation of the product of enzyme reaction 3,3-2H-cystathionine, which is not commercially available, was performed using cystathionine standard samples in the concentration range 0–5 μmol/L; the individual calibration points were 0, 0.25, 1 and 5 μmol/L. The calibration curve was obtained by linear regression; the peak area ratio (analyte/internal standard) was plotted against the analyte concentration. For the activity calculations, the measurable concentration of 3,3-2H-cystathionine in the blank sample (t = 0) was subtracted from the concentration produced by the enzyme reaction after incubation for 4 h.
Results
Human plasma exhibits CBS activity
With the employment of LC-MS/MS technology, we introduced a modification of an enzyme assay previously published (Janosik et al. 2009). We observed that incubation of control plasma samples according to the protocol described in “Materials and methods” resulted in the formation of 3,3-2H-cystathionine. The signals of the product of the enzyme reaction, 3,3-2H-cystathionine, determined in the assay mixture after incubation of control plasma and plasma from a CBS-deficient patient for 4 h are shown in Fig. 1a, b, respectively. The signals of 3,3-2H-cystathionine in the blank sample (control plasma, incubation time = 0 h) and internal standard are shown in Fig. 1c, d, respectively. The figures show a clearly higher signal of 3,3-2H-cystathionine determined in the assay using control plasma sample compared to the signals obtained by analysis of assay mixture with plasma from the CBS-deficient patient. The appearance of the signal of 3,3-2H-cystathionine in the blank sample could be partially explained by isotope contribution of unlabelled cystathionine presented in the analyzed sample to the signal of 3,3-2H-cystathionine. Nevertheless, despite the occurrence of the product signal in the blank sample, the assay performance allowed sufficient discrimination between plasma samples with normal and low or undetectable activities.
The LC-MS/MS chromatograms. Signal of 3,3-2H-cystathionine (retention time 15.4–15.5 min) was determined after incubation of the assay mixture with control plasma (a incubation time 4 h, c incubation time 0 h, sample blank), and a plasma from a CBS-deficient patient (b incubation time 4 h). d The signal of the internal standard 3,3,4,4-2H-labeled cystathionine. The peak with retention time 14.5 min belongs to an unknown compound, which is not a product of the CBS-catalyzed reaction
In order to confirm that the 3,3-2H-cystathionine formation was due to the presence of CBS in plasma, to elucidate the source of the enzyme in circulation and to validate the enzyme assay we performed experiments described in the following section.
Demonstration of CBS protein in human plasma by immunodetection and in silico searches
To demonstrate the presence of CBS antigen in plasma, we first performed western blotting after immunoprecipitation preceded by low abundant plasma protein enrichment. Multiple fractions including bands of approximately 60 and 45 kDa which were consistent with the presence of full-length and truncated forms of CBS were observed (data not shown). To confirm the identity of these fractions, we used gel digestion followed by peptide mass fingerprinting with MALDI-TOF MS detection; only abundant serum proteins such as albumin were identified with no peptide related to CBS being detected (data not shown). These data suggested that the fractions observed on western blots were due to the presence of cross-reactive highly abundant plasma proteins. In addition, we were unable to detect the CBS antigen by ELISA as this in-house assay had poor sensitivity (see Discussion).
As an alternative approach to demonstrate the presence of CBS in human plasma, we searched the available proteomic database Peptide Atlas (www.peptideatlas.org). As of February 1, 2010, the search only identified the presence of CBS peptides 2–18 and 414–439 in an experiment that employed cysteinyl-peptides enrichment (Liu et al. 2004), demonstrating that CBS is indeed present in normal human plasma but in only very low quantities.
Responsiveness of plasma CBS activity to S-adenosyl-L-methionine and pyridoxal 5′-phosphate
Since S-adenosyl-L-methionine (SAM) is an allosteric activator and pyridoxal 5′-phosphate (PLP) is a cofactor of human CBS, we tested whether the addition of these compounds into the assay mixture would increase the production of 3,3-2H-cystathionine.
We performed the assays in plasma samples (n = 6) according to the protocol described in “Materials and methods” with and without the addition of SAM (final concentration in the assay was 0.5 mmol/L) to the assay mixture. We observed that the addition of SAM to the assay mixture increased the production of 3,3-2H-cystathionine by a factor of 2.2 ± 0.2. Similarly, the assay was performed using control plasma samples (n = 6) with and without PLP added to the assay mixture. The production of 3,3-2H-cystathionine in the assay with PLP was 1.6 ± 0.1 times higher than without the cofactor added to the assay. The stimulatory effect of both compounds on the production of 3,3-2H-cystathionine in the assay gives further support to the hypothesis that human plasma contains CBS activity.
Origin of CBS in human plasma
To explore the hypothesis that CBS is released into plasma from organs with a high content of the enzyme, we determined the enzyme activity in plasma samples obtained from individuals with elevated biomarkers for liver (alanine aminotransferase, ALT and aspartate aminotransferase, AST), pancreatic (pancreatic amylase, p-AMS) and kidney (creatinine) disease. The results, shown in the Supplementary Table, clearly demonstrated an extremely elevated CBS activity (22463-231179 nmol/h/L) in plasma from individuals with increased ALT (2.4-15 μkat /L) and AST (2.1-14 μkat/L). Elevation of CBS activity was present also in the majority of plasma samples from individuals with elevated creatinine and p-AMS, respectively, although the plasma CBS activity increase was less striking than in patients with elevated plasma aminotransferase activities. These data are congruent with the hypothesis that CBS may be released into plasma from organs with a high content of this enzyme, mainly from liver.
Method validation
Assay linearity
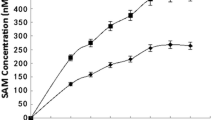
To determine the linearity of the assay we incubated the assay mixture for different time intervals (1, 2, 4 and 6 h). In addition, three experiments assays were performed with the addition of different plasma volumes to the assay mixture (5, 10 and 20 μL, respectively) and with an incubation time 4 h. Product formation was measured and plotted against different time points or varying plasma volumes, respectively. In both experiments, we observed linear relationships with correlation coefficients r 2 = 0.997 and r 2 = 0.999, respectively. The linearity of the time and dose relationships supports the presumption that the monitored compound, 3,3-2H-cystathionine, is a product of the enzyme reaction.
Intra- and Inter-day variation of the assay
The assay reproducibility was assessed by determination of intra- and inter-day coefficients of variation of the assay. For determination of the intra-day variation, the assay was performed with 5 identical control plasma samples in 1 day. For determination of the inter-day variation, the assays were performed using the control plasma on six separate days over an 11-month period. The mean of the activities were determined and coefficients of variation (in parentheses) for intra- and inter-day experiments were 354 nmol/h/L (1.4%) and 309 nmol/h/L (17 %), respectively. These data show that reproducibility of this assay was satisfactory and was consistent with the usually observed performance of enzyme assays.
Sample stability
Sample stability was assessed by assaying control plasma immediately following venepuncture and plasma separation, and after one and ten cycles of freezing and thawing. In addition, samples were also tested following incubation of plasma samples stored at 25°C for 6 h. No change in activity was seen after one freeze and thaw cycle. However, after ten cycles, a decrease of 13% in activity was observed. A similar decrease in activity (i.e., 11%) was also observed after incubation of the plasma for 6 h at room temperature. The activity changes resulting from freezing and thawing or sample storage at 25°C may have contributed to the significantly higher inter-day coefficient of variation.
Sample matrix effects
The validation experiments described were carried out on plasma with EDTA used as anticoagulant because the majority of archived samples from CBS-deficient patients available for this study were prepared with EDTA. However, to explore the influence of other sample matrices on CBS activity, we performed the assay using plasma (from one person) following blood being collected into EDTA- and heparin-containing tubes and serum collection tubes. The results showed similar activities in heparinized plasma samples and serum, whereas the activity in EDTA-treated plasma was substantially lower (∼22%) compared to the activity determined in the other two matrices. In addition, we evaluated the effect of haemolysis on CBS activity in plasma samples from a control subject and from two CBS-deficient patients with low (10 nmol/h/L) and undetectable enzyme activity, respectively. Haemolysis in one aliquot of EDTA blood was induced by a single cycle of freezing and thawing before centrifugation. Haemolysis had no measurable effect on plasma CBS activity.
CBS activities in plasma obtained from controls and CBS deficient patients
To assess the clinical utility of the plasma CBS assay in diagnosing patients with CBS deficiency, we measured activities in plasma samples obtained from control subjects, CBS-deficient patients and their parents. These samples were obtained from four European countries (see Supplementary Table and Fig. 2). The median plasma CBS enzyme activity from control subjects (n = 57) was 404 nmol/hour/L, the median plasma enzyme activity from pyridoxine nonresponsive CBS deficient patients (n = 26) was 0 nmol/hour/L. The median plasma enzyme activity from pyridoxine responsive patients (n = 28) was 16 nmol/h/L, higher than in the pyridoxine nonresponsive patients, but compared to the median of control subjects, it represented just 4% of the activity The median enzyme activities in the heterozygote parents (n = 9) was 547 nmol/h/L, showing no utility of the assay to recognize heterozygotes.
Because of the elevation of plasma CBS activity observed in samples from individuals with presumed liver disease (see “Origin of CBS in human plasma”) the most stable biomarker for the liver disease, γ-glutamyl transferase (GGT), was determined in archived samples analyzed in this study. The samples with elevated levels of GGT (higher than 0.8 μkat/L) showed increased CBS activity, i.e. higher than 1,300 nmol/h/L, and were therefore excluded from the control dataset. One pyridoxine responsive CBS deficient patient had an elevated plasma GGT (1.5 μkat/L) and CBS activity (770 nmol/h/L) and was also excluded from the presented dataset.
Discussion
Utilization of mass spectrometry for determination of CBS activity
The introduction of novel analytical techniques into routine diagnostic laboratories in the last decade has opened up the field for new approaches in enzyme assay methodology. Tandem mass-spectrometry in enzymology allows the use of substrates that are labeled with stable isotopes instead of radioisotopes; its utility has been discussed elsewhere (Gelb et al. 2006). In this study, we employed LC-MS/MS to design a novel CBS assay, utilizing isotopically labeled standard, 2,3,3-2H- serine. This method has excellent sensitivity and can detect nanomolar concentrations of the reaction product, 3,3-2H-cystathionine.
Compared to the previously described radiometric assay using 14C serine with a detection limit in the low micromolar range (Kopecka et al. 2010), the LC-MS/MS method is at least 2 orders of magnitude more sensitive and enables the determination of very low catalytic activities of CBS in human plasma, which would be undetectable by the established radiometric method. Using the radiometric method, we were able to detect product of the CBS activity, radioactive cystathionine, only in the sample from a patient with the most severe liver disease (data not shown).
Is there any measurable CBS activity present in human plasma?
Although CBS is an intracellular cytosolic enzyme, the breakdown of tissue containing high amounts of this enzyme may result in leakage of CBS protein into plasma, a phenomenon commonly observed in the case of other liver enzymes that are routinely measured in human plasma or serum (e.g., ALT, AST, GGT and others). Our study has shown that control human plasma is able to catalyze the formation of cystathionine with a median activity (404 nmol/h/L, i.e. 125 pkat/L) which is three to five orders of magnitude lower than activities of serum enzymes routinely used in clinical chemistry. Several lines of evidence demonstrate that this activity can be attributed to the presence of CBS in human plasma: (1) the absence of activities in plasma samples from pyridoxine nonresponsive CBS deficient patients and its presence in controls; (2) activation of plasma CBS by SAM and PLP by a factor of 2.2 and 1.6, respectively; (3) configuration of the detected reaction product, 3,3-2H-cystathionine, is in agreement with the previously observed CBS reaction mechanism (Banerjee and Zou 2005; Borcsok and Abeles 1982; Miles and Kraus 2004); this mechanism involves the formation of aminoacrylate intermediate with release of water containing one deuterium atom bound originally on the alpha-carbon of serine; and (4) the presence of CBS protein in normal human plasma was demonstrated by in silico search of a proteome database. Considering the specific activity of purified CBS protein (150 units/mg protein) and the plasma CBS activity in controls, we estimated that the quantity of CBS in human plasma is approximately in the range of nanograms per millilitre which does not allow the confirmation of the CBS presence in human plasma by western blotting.
Plasma CBS activity in CBS-deficient patients – utilization of the assay in diagnosis and study of CBS deficiency
In our study, we measured CBS activities in plasma from 54 patients with CBS deficiency and 57 control subjects. The range of activities in the plasma from pyridoxine nonresponsive CBS-deficient patients was clearly separated from the range obtained in plasma samples from control subjects. In contrast, the range of activities in plasma from pyridoxine responsive CBS-deficient patients overlaps with those from the control subjects.
To evaluate the use of the CBS assay in plasma for the diagnosis of CBS deficiency, we calculated the specificity and sensitivity of the assay. Since the lowest CBS activity in samples from control subjects was 67 nmol/h/L, this value was used as a threshold value for differentiating patients from controls. The sensitivity and specificity of the assay was excellent for pyridoxine nonresponders (i.e., sensitivity = 100% and specificity = 100%), while it was suboptimal for pyridoxine responders (i.e., sensitivity = 70% and specificity = 100%). These data suggest that the plasma CBS assay may be useful in detecting pyridoxine nonresponders, while its use for diagnosing responders may be limited.
The reason for the wide range of plasma CBS activities observed in pyridoxine responders (0-358 nmol/h/L) is unknown. This variability is caused not only by the underlying genetic defect but may result from a contribution of additional and yet unknown factors (as demonstrated by a wide range of plasma CBS activities in patients homozygous for the c.833T>C mutation; see Supplementary Table). It is at present unknown whether the CBS assay in plasma will be useful for predicting the phenotypic severity in pyridoxine responsive patients. The measurement of plasma CBS activity may be useful to evaluate or monitor the effectiveness of pyridoxine therapy. The latter hypothesis is supported by an experiment in which it was observed that plasma CBS activity in one patient increased from 4.5 to 55.9 nmol/h/L after the patient started pyridoxine therapy. However, in two other samples from patients responding to pyridoxine therapy, the activity was undetectable before as well as after the start of pyridoxine treatment (data not shown). Evaluation of the effects of pyridoxine therapy on plasma CBS activity would require further studies on patients before and on therapy.
To our knowledge, this is the first report demonstrating the presence of CBS activity in human plasma. This proof-of-principle study shows that very low catalytic activities of enzymes originating from liver can be measured in cell-free extracellular fluids. In summary, this paper highlights the possibility of measuring intracellular enzymes in plasma or serum using isotopically labeled substrates and LC-MS/MS instrumentation, and opens new possibilities for diagnosing other inborn errors of metabolism.
Abbreviations
- ALT:
-
Alanine aminotransferase
- p-AMS:
-
Pancreatic amylase
- AST:
-
Aspartate aminotransferase
- CBS:
-
Cystathionine β-synthase
- IEM:
-
Inborn errors of metabolism
- GGT:
-
γ-glutamyl transferase
- PLP:
-
Pyridoxal 5′-phosphate
- SAM:
-
S-adenosyl-L-methionine
References
Banerjee R, Zou CG (2005) Redox regulation and reaction mechanism of human cystathionine-beta-synthase: a PLP-dependent hemesensor protein. Arch Biochem Biophys 433(1):144–156
Bhagavan NV (2001) Medical biochemistry, 4th edn. Academic, London
Borcsok E, Abeles RH (1982) Mechanism of action of cystathionine synthase. Arch Biochem Biophys 213(2):695–707
Gaustadnes M, Rudiger N, Rasmussen K, Ingerslev J (2000) Intermediate and severe hyperhomocysteinemia with thrombosis: a study of genetic determinants. Thromb Haemost 83(4):554–558
Gelb MH, Turecek F, Scott CR, Chamoles NA (2006) Direct multiplex assay of enzymes in dried blood spots by tandem mass spectrometry for the newborn screening of lysosomal storage disorders. J Inherit Metab Dis 29(2–3):397–404
Janosik M, Sokolova J, Janosikova B, Krijt J, Klatovska V, Kozich V (2009) Birth prevalence of homocystinuria in Central Europe: frequency and pathogenicity of mutation c.1105C>T (p.R369C) in the cystathionine beta-synthase gene. J Pediatr 154(3):431–437
Kopecka J, Krijt J, Rakova K, Kozich V (2010) Restoring assembly and activity of cystathionine beta-synthase mutants by ligands and chemical chaperones. J Inherit Metab Dis (in press)
Liu T, Qian WJ, Strittmatter EF, Camp DG 2nd, Anderson GA, Thrall BD, Smith RD (2004) High-throughput comparative proteome analysis using a quantitative cysteinyl-peptide enrichment technology. Anal Chem 76(18):5345–5353
Miles EW, Kraus JP (2004) Cystathionine beta-synthase: structure, function, regulation, and location of homocystinuria-causing mutations. J Biol Chem 279(29):29871–29874
Mudd SH, Skovby F, Levy HL, Pettigrew KD, Wilcken B, Pyeritz RE, Andria G, Boers GH, Bromberg IL, Cerone R et al (1985) The natural history of homocystinuria due to cystathionine beta-synthase deficiency. Am J Hum Genet 37(1):1–31
Skovby F, Gaustadnes M, Mudd SH (2010) A revisit to the natural history of homocystinuria due to cystathionine beta-synthase deficiency. Mol Genet Metab 99(1):1–3
Stabler SP, Lindenbaum J, Savage DG, Allen RH (1993) Elevation of serum cystathionine levels in patients with cobalamin and folate deficiency. Blood 81(12):3404–3413
Acknowledgements
This work was supported by grant MZ0VFN2005 from the Ministry of Health of the Czech Republic. Institutional support was provided by the Research Project of the Ministry of Education of the Czech Republic (reg. no. MSM0021620806). The authors would like to express their gratitude to Ms. Alena Dutá for technical help, to Jitka Sokolová,MSc. for assistance in preparing the manuscript, to Dr. Jana Barcalová for the measurements of the liver enzymes, and to Květa Pelinková, MSc. for providing the plasma samples from individuals with presumed liver, pancreatic and kidney disease.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: Cornelis Jakobs
References to electronic databases:
http://www.chem.qmul.ac.uk/iubmb/enzyme/EC4/2/1/22.html
http://www.ncbi.nlm.nih.gov/entrez/dispomim.cgi?id=236200
Competing interest: None declared.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Table
CBS activity in human plasma. Plasma samples obtained from homozygotes and heterozygotes for CBS deficiency, controls and individuals with elevated aminotransferases, creatinine and pancreatic amylase. Reference ranges: ALT (alanine aminotransferase, 0.1–0.78 μkat/L), AST (aspartate aminotranferase, 0.1–0.72 μkat/L), creatinine (44–110 μmol/L), p-AMS (pancreatic amylase, 0.22–0.88 μkat/L). (+) patients receiving pyridoxine treatment at the time of sampling, (-) patients without pyridoxine treatment; CZ Czech Republic, NL Netherlands, IRE Ireland, UK United Kingdom, N.A. not available. (XLS 29 kb)
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Krijt, J., Kopecká, J., Hnízda, A. et al. Determination of cystathionine beta-synthase activity in human plasma by LC-MS/MS: potential use in diagnosis of CBS deficiency. J Inherit Metab Dis 34, 49–55 (2011). https://doi.org/10.1007/s10545-010-9178-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10545-010-9178-3