Abstract
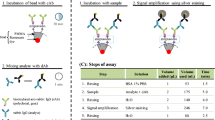
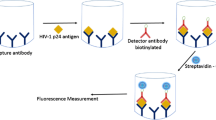
Accurate and affordable rapid diagnostic tests (RDTs) are indispensable but often lacking for many infectious diseases. Specifically, there is a lack of highly sensitive malaria RDTs that can detect low antigen concentration at the onset of infection. Here, we present a strategy to improve the sensitivity of malaria RDTs by using capillary-driven microfluidic chips and combining sandwich immunoassays with electroless silver staining. We used 5 μm fluorescent beads functionalized with capture antibodies (cAbs). These beads are self-assembled by capillary action in recessed “bead lanes”, which cross the main flow path of chips microfabricated in Si and SU-8. The binding of analytes to detection antibodies (dAbs) and secondary antibodies (2ndAbs) conjugated to gold nanoparticles (NPs) allows the formation of a silver film on the beads. Such silver film masks the fluorescent core of the bead inversely proportional to the concentration of antigen in a sample. We illustrate this method using the recombinant malaria antigen Plasmodium falciparum histidine-rich-protein 2 (rPfHRP2) spiked in human serum. This antigen was a recombinant HRP2 protein expressed in Escherichia coli, which is also the standard reference material. The limit of detection (LOD) of our immunoassay was found to be less than 6 ng mL−1 of rPfHRP2 within 20 min, which is approaching the desired sensitivity needed in the Target Product Profile (TPP) for malaria elimination settings. The concept presented here is flexible and may also be utilized for implementing fluorescence immunoassays for the parallel detection of biomarkers on capillary-driven microfluidic chips.





Similar content being viewed by others
References
Y. Arango, Y. Temiz, O. Gökçe, E. Delamarche, Appl. Phys. Lett. 112, 153701 (2018)
M. Cherbuin, F. Zelder, W. Karlen, Analyst 130 (2018)
J. T. Corthell, in Basic Mol. Protoc. Neurosci. Tips, Tricks, Pitfalls (2014)
S. Das, R.B. Peck, R. Barney, I.K. Jang, M. Kahn, M. Zhu, G.J. Domingo, Malar. J. 17, 1 (2018)
Foundation for Innovative New Diagnostics, HRP2 recombinant panel for malaria diagnostic tests (2017)
A. Jimenez, R.R. Rees-Channer, R. Perera, D. Gamboa, P.L. Chiodini, I.J. Gonzalez, A. Mayor, X.C. Ding, Malar. J. 16, 128 (2017)
D. Juncker, H. Schmid, U. Drechsler, H. Wolf, M. Wolf, B. Michel, N. De Rooij, E. Delamarche, Anal. Chem. 74, 6139 (2002)
P.M. Lackie, Histochem. Cell Biol. 106, 9 (1996)
L. Lafleur, D. Stevens, K. McKenzie, S. Ramachandran, P. Spicar-Mihalic, M. Singhal, A. Arjyal, J. Osborn, P. Kauffman, P. Yager, B. Lutz, Lab Chip 12, 1119 (2012)
R. Liu, Y. Zhang, S. Zhang, W. Qiu, Y. Gao, Appl. Spectrosc. Rev. 49, 121 (2014)
M.L. Mcmorrow, M. Aidoo, S.P. Kachur, Clin. Microbiol. Infect. (2011)
N.M. Pham, W. Karlen, H.-P. Beck, E. Delamarche, Malar. J. (2018a)
N.M. Pham, S. Rusch, Y. Temiz, R.D. Lovchik, H.-P. Beck, W. Karlen, E. Delamarche, Biomed. Microdevices 20(41), 41 (2018b)
Program for Appropriate Technology in Health, Target Product Profile : Point-of-Care Malaria Infection Detection Test (2014)
D.Y. Stevens, C.R. Petri, J.L. Osborn, P. Spicar-mihalic, K.G. Mckenzie, P. Yager, T. Int, Conf. Miniaturized Syst. Chem. Life Sci. 1768 (2008)
Y. Temiz, E. Delamarche, J. Micromech. Microeng. 24, 097001 (2014)
The malERA Consultative Group on Diagnoses and Diagnostics, PLoS Med. 8, e1000396 (2011)
World Health Organization, Universal Access to Malaria Diagnostic Testing: An Operational Manual (2011)
World Health Organization, World Malaria Report 2017 (2017)
World Health Organization, Terms of Reference for the Strategic Advisory Group of Experts on In Vitro Diagnostics (SAGE IVD) (2018a)
World Health Organization, Public Report: One Step Test for Malaria Pf / Pv Ag MERISCREEN Malaria Pf / Pv Ag (2018b)
World Health Organization, Foundation for Innovative Diagnostics, Centers for Disease Control and Prevention, Malaria Rapid Diagnostic Test Performance Round 2 (2009)
G. Xu, D. Nolder, J. Reboud, M.C. Oguike, D.A. van Schalkwyk, C.J. Sutherland, J.M. Cooper, Angew. Chem. Int. Ed. 55, 15250 (2016)
M. Zimmermann, H. Schmid, P. Hunziker, E. Delamarche, Lab Chip 7, 119 (2007)
Acknowledgements
Ngoc M. Pham received an Engineering for Development Scholarship from ETH Global and the Sawiris Foundation for Social Development. Walter Karlen is supported through the Swiss National Science Foundation professorship award 150640 “Intelligent point-of-care monitoring”. Yuksel Temiz and Emmanuel Delamarche thank Elisa Hemmig, Robert Lovchik and Onur Gökçe for discussions and Walter Riess and the IBM Research Frontiers Institute for their continuous support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 833 kb)
Rights and permissions
About this article
Cite this article
Pham, N.M., Rusch, S., Temiz, Y. et al. Immuno-gold silver staining assays on capillary-driven microfluidics for the detection of malaria antigens. Biomed Microdevices 21, 24 (2019). https://doi.org/10.1007/s10544-019-0376-y
Published:
DOI: https://doi.org/10.1007/s10544-019-0376-y