Abstract
Minimal residual disease (MRD), a measure of residual cancer cells, is a concept increasingly employed in precision oncology, touted as a key predictive biomarker to guide treatment decisions. This paper critically analyzes the expanding role of MRD as a predictive biomarker in hematologic cancers. I outline the argument for MRD as a predictive biomarker, articulating its premises and the empirical conditions that must hold for them to be true. I show how these conditions, while met in paradigmatic cases of MRD use in cancer, may not hold across other cancers where MRD is currently being applied, weakening the argument that MRD serves as an effective predictive biomarker across cancer medicine.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Minimal or measurable residual disease (MRD) is a concept increasingly employed in precision oncology, an approach which aims to use novel biomarkers to inform ‘personalized’ treatment of cancer. MRD refers to the presence of a small quantity of malignant cells following initial treatment and is often touted as a key biomarker and one of the most important predictors of disease relapse in several cancers. As a result, MRD use is expanding both as a surrogate outcome measure in clinical trials and as a predictive biomarker to guide treatment decisions. This paper focuses on the latter use of MRD. The concept of MRD intersects with a number of epistemic and ontological issues in the philosophy of cancer and precision oncology (Plutynski 2018, 2022; Pradeu et al. 2023). However, a philosophical analysis of MRD as a predictive biomarker, with a focus on challenges translating this concept from research to practice, is currently lacking. This paper seeks to fill this lacuna by critically analyzing the expanding role of MRD as a predictive biomarker in cancer medicine.
My approach involves reconstructing the argument for MRD as a predictive biomarker, articulating its premises and then examining the conditions required for each premise to be true. Methodologically, this bears a loose resemblance to Okasha’s (2024) recent analysis of the conditions necessary to justify a certain scientific conceptualization of cancer, in his case as an instance of multilevel selection. In my case, I analyze the conditions necessary to justify a particular clinical conceptualization of cancer, namely as a disease in which MRD is an effective predictive biomarker. My approach also follows influential examples in the philosophy of medicine where argument reconstruction is used to examine assumptions underlying important claims about, for example, types of medical evidence such as randomized trials (e.g., Cartwright 2010), in order to highlight the conditions required to warrant those claims. Here, the result is to highlight the conditions required for MRD to serve as an effective predictive biomarker in hematologic cancers. These are, to be sure, still empirical conditions which may or may not be met in particular cancers. This paper, therefore, should not be read as an attempt to deductively prove or refute the utility of a biomarker on the basis of philosophical argumentation; such an approach would clearly be misguided. Rather, it is an attempt to clearly state and scrutinize the assumptions behind a widely and increasingly employed biomarker in cancer medicine in order to help guard against potentially unwarranted inferences.
Bearing in mind this methodological disclaimer, I proceed as follows. The first section begins with a brief historical introduction to the concept of MRD in acute leukemia before turning to more general ontological questions regarding MRD’s role in cancer biology and status as a privileged biomarker in oncology. The second section outlines the argument that MRD is a predictive biomarker in cancer, articulating premises which, although rarely explicitly stated in the literature, are required for its success. The next two sections critically analyze the necessary conditions for each premise, examining the empirical facts about cancer biology and cancer treatment that must obtain for each premise to be true. While these conditions may be established in paradigmatic cases of MRD use in oncology, such as some types of acute leukemia, I present reasons to doubt that they hold in other cancers, weakening the argument that MRD is an effective predictive biomarker across cancer medicine. In so doing, I not only establish what is required for successful use of MRD in practice but also explain why MRD is unlikely to be successful as a predictive biomarker in several cancers where it is currently being applied.Footnote 1 The contribution of this paper is thus twofold: first, it helps articulate often unstated assumptions behind use of MRD as a guide to cancer treatment, highlighting the conditions needed for its successful use; and second, it cautions against a trend towards uncritical adoption of MRD as a predictive biomarker across all cancers.
A brief history of MRD in acute leukemia
The history of MRD is closely linked to advances in the treatment of acute leukemia and technologies for measuring malignant cells. In 1948, Sidney Farber and colleagues from the Boston Children’s Medical Center described how the synthetic antifolate compound aminopterin (chemically related to the drug methotrexate, still in use today) could induce temporary remissions in children with acute leukemia. Acute leukemia, at the time, was a universally fatal disease, making this a remarkable finding. Bone marrow examination following treatment revealed ‘disappearance of the leukemic cells’ (Farber et al. 1948). Unfortunately, despite achieving morphological remission all patients invariably relapsed. This suggested the presence of residual disease not detected at the time of remission.Footnote 2
Farber (1949) was careful not to interpret his observations as indicating a potential cure. However, in the decades that followed cure became a viable goal in the treatment of childhood acute leukemia, particularly acute lymphoblastic leukemia (ALL). This possibility of cure was based on the use of combination chemotherapy alongside central nervous system-directed therapy in a regimen known as ‘total therapy’, which involved high doses and long durations of treatment aimed at ‘total eradication of the disease’ (George et al. 1968; Simone et al. 1972). ‘Total therapy’ was remarkably effective: within two decades of Farber’s initial report, approximately half of children with ALL could be cured (Aur et al. 1971; Pui and Evans 2013). The success of this approach helped bolster a paradigm of cancer treatment involving intensive, multi-agent chemotherapy aimed at total disease eradication, with far reaching impact beyond ALL.
MRD was a key part of this story. Despite the early successes of ‘total therapy’, detecting residual leukemic cells to help better guide treatment remained a pressing concern. In 1981, Joseph Simone, then director of the St. Jude’s Children’s Research Hospital and pioneering figure in the treatment of childhood ALL remarked:
The most urgent need of the 1980s, in my view, is a method for detecting minimal residual disease in the bone marrow of patients who are in remission of ALL. If we had the capacity for detecting one leukemia cell in a thousand or a million or more, we would be able to measure the effectiveness of therapy given during remission. Currently, of course, we must wait for relapse to occur and we have no clue as to when resistance may have developed…
That same year, Kenneth Bradstock, George Janossy and colleagues (1981) published a landmark article, often cited as the first example of MRD measurement using immunofluorescence microscopy. This study showed how patients with ALL who achieved morphological remission could still have residual leukemic cells—as low as one cell in two hundred—which were not identifiable by conventional morphological analysis, but which could be identified using novel immunofluorescence techniques. These techniques built on advances in cancer immunology with the characterization of cell membrane surface markers in acute leukemia and the development of monoclonal antibodies against these markers (Greaves and George 1978). This allowed for identification of residual leukemic cells based on aberrant expression of cell surface markers using immunofluorescence rather than relying on less sensitive morphological analysis. Patients harbouring residual leukemic cells were shown to have a propensity for early relapse, further supporting the idea that treatment should be aimed at eradicating these previously undetectable malignant cells (Campana 1994). For the first time, a biomarker was available to measure depth of response to therapy and predict disease relapse.
During the subsequent decade, technologies for detection of leukemic cells were increasingly refined with the advent of multiparameter flow cytometry, which allowed for automated counting of cells on orders of magnitude higher than microscopic techniques (Campana et al. 1990). This enabled MRD assessment to be developed into a clinical assay and used as a biomarker in major trials and subsequently clinical practice. Modern flow cytometers used for MRD assessment in ALL can now count over one million cells with the ability to detect a single leukemic cell in over ten thousand cells. Parallel advances in molecular technologies for detecting nucleic acids, such as quantitative polymerase chain reaction and next generation sequencing, offered alternate methods of MRD detection. These have even higher sensitivities than flow cytometry, detecting quantities of DNA/RNA equivalent to a single leukemic cell in a million cells (Saygin et al. 2022). Now, forty years later, Simone’s (1981) goal of detecting ‘one leukemia cell in a thousand or a million or more’ has been achieved.
These methods for measuring MRD, along with mounting evidence for its importance in predicting clinical outcomes (Brüggemann et al. 2006; Stow et al. 2010), led to MRD assessment becoming part of the standard of care in pediatric and adult ALL. In ALL, MRD status has superseded all other prognostic factors and serves as the most important biomarker for measuring response to treatment and individualizing therapy. As the authors of a recent study conclude: ‘Minimal residual disease is a “biomarker” of disease in the powerful sense that MRD is the disease’ (Berry et al. 2017; emphasis in original). In this way, MRD seems to transcend the conventional sense of biomarker as a mere ‘indicator’ of a pathogenic process (FDA-NIH 2016). For example, it seems to differ from other commonly used biomarkers, such as glycosylated hemoglobin A1c in diabetes, which serves only as an indirect indicator of insulin deficiency or resistance, the basic pathogenic process behind diabetes. Rather, MRD is taken to measure the pathogenic process—the cancer—itself and is interpreted by clinicians as providing ‘direct’ quantification of the presence or absence of the disease they are treating. Or so it seems.
The fact that this novel biomarker, which measures a minute quantity of cancer cells or nucleic acids, is used to define a state of ‘residual disease’ serves as an interesting case study for philosophical debates over definitions of disease and ‘drawing the line’ between health and disease (e.g., Schwartz 2007, 2014; Plutynski 2018, pp. 61–87; see also Rogers and Walker 2017). As suggested by very term itself, states of ‘residual disease’, while previously designated ‘remission’ or absence of disease, are now frequently interpreted as disease states. This interpretation is common, as illustrated by the above view that ‘MRD is the disease’ in ALL (Berry et al. 2017). One might interpret this shift in line-drawing in different ways, depending on one’s philosophical view of disease. For the naturalist (e.g., Schwartz 2007), this shift might be understood as resulting from finer grained assessments of dysfunction paired with new evidence of negative consequences. For the constructivist (e.g., Kukla 2022), it might instead be understood as a strategic shift driven by pragmatic considerations, namely that considering patients previously deemed ‘in remission’ to have disease confers certain benefits. For both, however, the shift is contingent on a range of technical factors that enabled MRD assessment and made it possible to designate such states as pathological in the first place.
Whether such a shift is warranted across all cancers—whether a small quantity of residual cancer cells constitutes a disease—is debatable. In some cases, one might reasonably question whether MRD is in fact a disease state or rather is better understood as a risk factor for disease, more akin to a pre-cancerous state, for example (e.g., Schwartz 2014). Such determinations may require a closer look at the consequences associated with MRD, which in turn requires examining specific assumptions it makes about cancer biology, or considering the benefits and harms of intervening in MRD, the focus of the subsequent sections. One philosophical upshot of my argument that MRD often fails as a predictive biomarker may be that some patients with ‘residual disease’ should not be considered to have disease, at least not according to influential naturalist and constructivist accounts of line-drawing, given lack of evidence of negative consequences (Schwartz 2007), or lack of strategic benefit for patients (Kukla 2022). For now, however, I set aside these ontological questions about MRD’s status as a disease, which is peripheral to my main argument, and instead focus my attention on MRD’s role as a predictive biomarker.
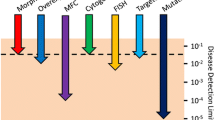
MRD’s status as a privileged biomarker, perceived as giving ‘direct’ access to disease and redefining the line between presence and absence of disease, has its origins in acute leukemia but has been influential throughout cancer medicine. Use of MRD has expanded across a range of cancers, particularly hematologic cancers, where it is increasingly employed as a prognostic and predictive biomarker as well as a surrogate outcome measure in clinical trials (Fig. 1).Footnote 3
Percentage of phase 2 and 3 clinical trials in hematologic cancers registered at ClinicalTrials.gov in which ‘Minimal Residual Disease’ or ‘Measurable Residual Disease’ was listed as an outcome measure. Data extracted from ClinicalTrials.gov on November 10, 2021. ALL Acute Lymphoid Leukemia, AML Acute Myeloid Leukemia, MM Multiple Myeloma, CLL Chronic Lymphocytic Leukemia, MRD Minimal or Measurable Residual Disease
Chronic lymphocytic leukemia (CLL), a cancer of B lymphocytes, and multiple myeloma, a cancer of plasma cells, are two diseases in particular which have seen growing applications of MRD to guide treatment. It is important to note, however, that these diseases are biologically and clinically quite different from acute leukemia.
Firstly, both CLL and myeloma have well-described pre-cancerous phases: Monoclonal B-cell Lymphocytosis (MBL) in the case of CLL and Monoclonal Gammopathy of Undetermined Significance (MGUS) in the case of myeloma. These pre-cancerous conditions are prevalent and generally have low rates of progression to cancer—for example, MGUS is found in over five percent of individuals above age seventy, with a risk of progression to myeloma of approximately one percent per year (Kyle et al. 2010). Here again, drawing the line between pre-cancer and cancer—between MGUS and myeloma or between MBL and CLL—rests on a number of parameters (clinical, laboratory and radiologic), which serve as indicators of adverse outcomes, i.e., meeting a certain threshold of negative consequences (Schwartz 2007).
Secondly, unlike acute leukemia, both CLL and myeloma have a more indolent natural history and can frequently be monitored upon progression without urgent need for therapy. Most patients maintain high response rates in successive lines of therapy, contributing to longer overall survival which exceeds ten years in many patients, leading some to consider CLL and myeloma chronic diseases (Rajkumar 2008). Lastly, CLL and myeloma most often occur in older patients who are typically less tolerant of intensive regimens aimed at ‘total’ disease eradication as in the case of ALL. I return to these differences in the following sections. Next, I outline the argument that MRD is an effective predictive biomarker.
MRD as a predictive biomarker
What is a predictive biomarker and what criteria need to be met for MRD to be considered one? According to the US Food and Drug Administration (FDA) and National Institutes of Health (NIH), a predictive biomarker identifies individuals who are likely ‘to experience a favorable or unfavorable effect from exposure to a medical product’ (FDA-NIH 2016, p. 19). This contrasts with a prognostic biomarker, which identifies the ‘likelihood of a clinical event, disease recurrence or progression’ (p. 23), and is not defined with reference to a specific medical product or intervention. Predictive biomarkers play a key role in precision oncology by identifying individuals expected to respond to specific targeted therapies, thus informing ‘personalized’ treatment decisions. In other words, a predictive biomarker serves as an indication for, i.e., a reason to use, a specific treatment. One way of conceptualizing precision oncology is as an approach centred on expanding the set of predictive biomarkers to guide individualized treatment of cancer (Chin-Yee 2024). There is a growing push to use MRD as a predictive biomarker across a range of hematologic cancers.
The following argument attempts to make the FDA-NIH definition of a predictive biomarker more precise while also clarifying what is required for MRD to be considered an effective predictive biomarker in cancer.
Predictive Biomarker Argument (PBA).
Definition: X is a predictive biomarker for treatment T iff X is an indication for T.
Premise 1: MRD implies a probability of cancer relapse above a certain threshold, P(R) > θ.Footnote 4
Premise 2: When the probability of cancer relapse is above a certain threshold, P(R) > θ, treatment T is indicated.
Conclusion: MRD is a predictive biomarker for T.
The PBA makes clear the premises supporting the conclusion that MRD is a predictive biomarker for a given treatment T, namely that MRD implies a high probability of cancer relapse (Premise 1, P1), and that treatment T can effectively prevent or treat cancer relapse (Premise 2, P2).
In the case of ALL, MRD meets the requirements to be considered a predictive biomarker for specific interventions. Presence of MRD (or ‘MRD positivity’) in ALL implies a high risk of cancer relapse, and cancer relapse can be effectively prevented with specific treatments, such as intensification of chemotherapy, targeted drugs or stem cell transplantation, i.e., specific treatments are indicated when risk of relapse is high (Brown et al. 2022). Conversely, absence of MRD (or ‘MRD negativity’) in ALL, implies a low risk of relapse such that these specific treatments are not indicated, i.e. treating would expose patients to unnecessary harms and constitute what oncologists call ‘overtreatment’. I take both cases to exemplify uses of MRD as a predictive biomarker. MRD’s success as a predictive biomarker in ALL is based on the truth of P1 and P2 in this particular disease, for particular therapies.
We are now in a position to look at the conditions required for each premise of the PBA and to evaluate whether they obtain different cancers where MRD is being applied. The next two sections examine the conditions for P1 and P2, presenting reasons to doubt that they hold in all hematologic cancers, thus weakening the argument that MRD serves as an effective predictive biomarker across cancer medicine.
MRD does not imply cancer relapse
P1 in the PBA states that MRD implies a high probability of cancer relapse. In this section, I show how the truth of this premise is dependent on two conditions. The first condition has to do with a ‘stemness property’ of the cells constituting MRD:
Condition 1: The residual clone constituting MRD exhibits an intrinsic stemness property which enables it to independently recapitulate a cancer.
The second condition relates to the fitness of the cells constituting MRD:
Condition 2: The residual clone constituting MRD has a strong selective advantage relative to normal cells sufficient to cause cancer relapse.
I examine each of these in turn, showing how these conditions are jointly necessary but unlikely to be met in hematologic cancers where MRD is being used, contrasting the successful case of ALL with examples from CLL and myeloma.
Condition 1: intrinsic stemness property
Condition 1 (C1) is based in cancer stem cell (CSC) theory, which holds that a small number of self-renewing stem cells are responsible for cancer initiation, progression, and relapse. This view has been influential in explaining cancer and informing treatment approaches (Laplane 2016, 2018). While there is ongoing debate among philosophers over the explanatory merits of CSC theory and its distinctiveness from other models of cancer (Plutynski 2019), it nevertheless remains a well-accepted view among scientists, especially in hematologic cancers (Clevers 2011; Batlle and Clevers 2017). Research in acute and chronic leukemias was key to establishing the existence of CSCs, which in turn informs how these diseases are explained and treated (Dick 2008). For example, the ability of CSCs to survive chemotherapy and regenerate a tumour explained the high rates of relapse in acute leukemia following initial therapy. The concept of CSCs is thus closely linked to the concept of MRD; cancer cells that persist after initial treatment, measured as MRD, are understood as being enriched for CSCs (Ghiaur et al. 2012). The assumption is that the cells constituting MRD exhibit a stemness property, which explains why a very small number of cells are sufficient to cause relapse and, therefore, why MRD positivity is such an adverse prognostic biomarker.
To fully understand what this condition demands, however, requires further details on the precise nature of this stemness property. As Laplane (2016, p. 91) points out, CSC theory is ‘laden with conceptual ambiguities’. One important source of ambiguity is that there are different ways of interpreting the property of stemness, which carry different implications for how best to treat cancer. Laplane (2016, 2018) outlines four different senses of stemness, each placing different explanatory weight on cell-intrinsic vs. extrinsic factors:
-
1.
Categorical: stemness is purely intrinsic.
-
2.
Dispositional: stemness is an intrinsic property whose expression depends on extrinsic stimuli from the microenvironment.
-
3.
Relational: stemness is an extrinsic property induced and controlled by the microenvironment.
-
4.
Systemic: stemness is an extrinsic property controlled at the cell population level.
The stemness property required for the truth of P1 stresses cell-intrinsic factors, relying on either a categorical or dispositional notion of stemness. According to the categorical conception of stemness, the factors that determine a CSC’s identity are intrinsic to that cell and not dependent on the microenvironment, whereas the dispositional conception acknowledges the necessity of some extrinsic stimuli in addition to cell-intrinsic factors.
Each view says something different about MRD’s causal role in cancer relapse and thus its utility as a predictive biomarker. In cases of MRD positivity, a categorical stemness property would mean that intervening to eradicate MRD is both necessary and sufficient to prevent cancer relapse. A dispositional stemness property would mean that eradicating MRD positivity is sufficient to prevent cancer relapse but may not be necessary, as other strategies targeting extrinsic factors may be equally effective. According to both views, MRD positivity would be expected to serve as a useful predictive biomarker, indicating possible benefit of more intensive treatment aimed at MRD eradication, which is at least sufficient to prevent cancer relapse. Likewise, MRD negativity, qua absence of CSCs, also serves as a useful predictive biomarker according to both categorical and dispositional conceptions given the necessity of cell-intrinsic factors for stemness in both views. Patients who are MRD negative would not be expected to relapse, making further treatment unnecessary and potentially harmful. Therefore, according to both categorical and dispositional conceptions of stemness, MRD plays a key causal role in cancer relapse, supporting the truth of P1 in the PBA. For simplicity, I refer to this in C1 as an ‘intrinsic stemness property’ which I take as covering both categorical and dispositional conceptions.
If, however, stemness is instead an extrinsic property, be it controlled by the microenvironment (relational) or at the cell population or tissue level (systemic), then all bets are off. For cases of MRD positivity, MRD eradication becomes neither necessary nor sufficient to prevent cancer relapse. Firstly, strategies targeting extrinsic factors could prove equally or more effective than targeting MRD. Secondly, these extrinsic factors could potentially generate new CSCs, leading to cancer relapse even if MRD were successfully eradicated.Footnote 5
For the same reasons, MRD negativity may no longer predict a low risk of cancer relapse. An extrinsic stemness property thus undermines MRD’s causal role in cancer relapse and the truth of P1: MRD no longer implies cancer relapse, as extrinsic factors can supervene to prevent relapse in cases of MRD positivity or produce relapse in cases of MRD negativity. The truth of P1 in the PBA, and therefore MRD’s success as a predictive biomarker, requires an intrinsic stemness property (C1) rather than an extrinsic stemness property.
Research in acute leukemia supports an intrinsic stemness property of a so-called ‘leukemic stem cell’. Seminal experiments by John Dick and colleagues in Toronto demonstrated that a specific subpopulation of leukemic cells bearing surface markers characteristic of stem cells was both necessary and sufficient to recapitulate acute myeloid leukemia in a mouse model (Lapidot et al. 1994), findings interpreted as establishing the existence of a leukemic stem cell (Dick 2008). The idea of a leukemic stem cell with an intrinsic capacity to cause disease relapse became influential in the field and was quickly translated to the clinic. Studies demonstrated that patients with acute leukemia who had persistent leukemic stem cells following treatment, measured as MRD positivity, had high rates of relapse and poor overall survival, reinforcing the notion that eradicating leukemic stem cells was required to treat the disease (van Rhenen et al. 2005). Indeed, this strategy of aiming for MRD negativity as a measure of eradication of residual leukemic stem cells with an intrinsic stemness property has been largely successful in ALL, as discussed above. What is true of ALL is also increasingly the case in acute myeloid leukemia with the advent of newer molecular methods for MRD assessment allowing for more accurate and sensitive detection of specific clones responsible for disease relapse (Schuurhuis et al. 2018). In this way, acute leukemia provides a model for MRD use in oncology and illustrates how attributing an intrinsic stemness property to the residual cancer cells measured by MRD assays is a key part of this successful application.
Challenges to condition 1
While C1 is well-established in some cancers, such as acute leukemia, it encounters challenges in other cancers. These challenges can come in two forms: evidence that stemness is extrinsic and evidence casting doubt on the extent to which stemness is intrinsic.
The former would offer the strongest challenge to C1, most severely undermining the truth of P1 that MRD implies a high risk of cancer relapse, and therefore MRD’s utility as a predictive biomarker. Recall, if stemness were an extrinsic property, eradicating MRD would be neither necessary nor sufficient to prevent disease relapse. There is indeed evidence for an extrinsic stemness property in many biological systems, including some solid tumours such as breast cancer, where cancer cells have demonstrated an ability to de-differentiate and produce new CSCs.Footnote 6 Less evidence is available from hematologic cancers, although the bone marrow microenvironment is known to exert control over hematopoietic stem cells and researchers have demonstrated that stemness can be ‘reprogrammed’ in committed blood cells through extrinsic factors (e.g., Ridell et al. 2014). At the very least, this raises the possibility of extrinsic stemness, casting doubt whether C1 holds across all cancers.
More evidence is available to challenge the extent to which stemness is intrinsic. Consider CLL, for example, where MRD is increasingly used as a predictive biomarker to guide treatment (Thompson and Wierda 2016). Unlike in acute leukemia, a CSC has yet to be definitively identified in CLL. However, there are strong reasons to suspect that, if such a cell were found, it would rely more heavily on extrinsic factors, owing to the well-established roles of signalling in the bone marrow and antigenic stimulation in lymphoid organs for disease initiation and progression (Burger and Gribben 2014). As Purroy and Wu (2017) remark: ‘CLL is considered a prototype of a microenvironment-dependent tumour in which neoplastic cells coevolve together with host immune cells within specific tissue microenvironments, such as bone marrow or lymph nodes.’
The importance of extrinsic factors in CLL is supported by experiments transplanting hematopoietic stem cells from patients with CLL into a mouse model (Kikushige et al. 2011). This gave rise to monoclonal B-cell populations in some mice (analogous to the well-characterized CLL precursor MBL in humans) but did not progress to CLL. These observations can be interpreted in different ways. While some saw this as a demonstration of a cell-intrinsic capacity to initiate a precursor condition to CLL—and therefore as proof of a CSC in CLL—it can also be interpreted as the transplanted cells being insufficient to cause disease on their own, and reliance on extrinsic signals from the microenvironment to produce a CLL phenotype (Bertilaccio et al. 2013). In other words, these cells failed to exhibit intrinsic stemness qua ability to independently recapitulate the cancer, CLL. This finding is unsurprising, as the need for extrinsic signals, such as chronic antigenic stimulation, plays a key role in explaining why only a minority of individuals with MBL—who make up over ten percent of the population older than sixty—progress to CLL (Rawstron et al. 2008).
This evidence, to be sure, is only indirect—while it highlights the need for extrinsic factors, it does not definitively show stemness to be extrinsic. Nor is it incompatible with stemness being, to a certain extent, intrinsic as in a dispositional stemness property, according to which MRD eradication might still be sufficient (although not necessary) to prevent cancer relapse. Nonetheless, these findings do undermine the commonly held idea driving MRD use that cell-intrinsic factors dominate. This should lead us to temper claims about MRD’s virtues as a predictive biomarker. If one claims that MRD eradication is sufficient to prevent relapse, they should produce evidence that extrinsic factors cannot circumvent this strategy; if one claims that MRD eradication is necessary, they should produce evidence that targeting extrinsic factors is not also a viable strategy. This evidence has yet to be produced for several cancers where MRD is touted as a key biomarker.
On the contrary, the latter claim is directly challenged by recent advances in the treatment of CLL. Recognition of the role of extrinsic factors in CLL initiation and progression motivated approaches targeting signalling in the microenvironment. The most successful of these are Bruton kinase inhibitors, such as ibrutinib, which target antigenic signalling via the B-cell receptor. This class of drugs is now widely used for the frontline treatment of CLL, leading to significant improvement in clinical outcomes. Notably, in contrast to chemotherapeutic approaches, ibrutinib’s efficacy is not tied to its ability to eradicate MRD; in fact, it generates lower rates of MRD negativity compared to chemotherapy despite improving survival (Shanafelt et al. 2019; Wang et al. 2021). This example further highlights the pitfalls of expanding MRD’s use from predictive biomarker to surrogate endpoint in clinical trials, a growing trend in need of close scrutiny (Fig. 1).Footnote 7 For the purposes of this paper, which focuses on MRD’s role as a predictive biomarker, CLL provides an example which casts doubt on whether C1 holds across different cancers, which in turn calls into question the truth of P1 that MRD implies a high risk of cancer relapse.
Condition 2: strong selective advantage
Condition 2 (C2) states that the residual clone constituting MRD has a strong selective advantage relative to normal cells sufficient to cause cancer relapse. Whereas C1 deals with the more basic capacity for residual cells to recapitulate cancer by possessing an intrinsic stemness property, C2 has to do with the cancer cells’ ability to proliferate at a sufficient rate to cause relapse. Another way of stating C2 is that the residual cancer cells have a high relative fitness compared to normal cells (wC > > wN), where ‘high’ means generating selective pressures sufficient to cause clinically significant growth of the malignant clone in a relevant time period.
Prima facie, C2 seems uncontroversial. After all, uncontrolled proliferation is a ‘hallmark’ of cancer, attributed to a fitness advantage on the part of the malignant clone, owing to alterations in cellular replication machinery, cooptation of host resources, evasion of immune defenses, among other factors (Hanahan and Weinberg 2011). Indeed, selection acting on this fitness advantage has been the dominant explanation for clonal evolution in cancer (Nowell 1976). Recently, the development of clonal hematopoiesis, which precedes the development of several hematologic cancers such as acute leukemia, has been shown to result from a strong fitness advantage conferred by specific mutations which increase self-renewal relative to normal hematopoietic stem cells (Watson et al. 2020).
Selective pressures are further introduced by various treatments which increase the relative fitness of cells exhibiting drug resistance mechanisms. MRD, usually measured following initial therapy, is often claimed to be enriched for resistant cells that have a strong selective advantage.Footnote 8 That C2 should obtain across most, if not all, cancers therefore, seems highly plausible. One would expect residual cancer cells to have a high relative fitness, proliferating at rate sufficient to cause disease relapse.Footnote 9 This is indeed the case in ALL, where the time from MRD positivity to clinical relapse for most patients is less than one year (Raff et al. 2007).
Challenges to condition 2
Increasingly, however, evolutionary processes other than selection have come into focus as playing key roles in cancer (Merlo et al. 2006). Rather than clonal evolution in cancer being driven by selection alone, studies have shown neutral evolution to be common across a range of tumours (Williams et al. 2016; Caravagna et al. 2020). Neutral evolution refers to evolution in the absence of selection, whereby changes in cell lineages are explained by stochastic processes, i.e., genetic drift, rather than differences in fitness. In finite populations, the effect of genetic drift is inversely related to the effective population size (Ne) such that when the selection coefficient (s = wC − 1) is significantly less than 1/Ne genetic drift rather than selection becomes the dominant process that shapes population dynamics. Lyne et al. (2021) illustrate this effect by modelling clonal evolution in hematologic cancers, showing how genetic drift dominates in populations with a small number of stem cells and how it produces a pattern of clonal evolution consistent with what is seen in certain types of leukemia.
Genetic drift’s inverse relationship with population size may be particularly relevant to the dynamics of MRD, given that MRD measures very small populations of cancer cells. As Lyne et al. (2021) emphasize, we should not always assume that selection is the dominant process driving clonal evolution. Recognizing the role of other processes, such as genetic drift, can have important clinical consequences. If the relative fitness of the residual cancer cells is not sufficiently high to outweigh the effects of genetic drift (1/Ne >> wC − 1), there will not be a strong selective advantage for the cancer cells constituting MRD, undermining their ability to cause cancer relapse. In other words, in order for MRD to imply a high risk of cancer relapse as per P1 in the PBA, C2 must hold—there must be a strong selective advantage for the residual clone.
While the dynamics of MRD have not been directly studied in hematologic cancers, the natural history of more indolent cancers such as CLL suggests that C2 may not always hold. A recent study of the clonal dynamics of CLL demonstrated how growth is not always exponential as one might expect for a clone with a strong selective advantage but often showed a logistic growth pattern towards a finite carrying capacity (Gruber et al. 2019). This carrying capacity was hypothesized to result from competition between clones produced by processes of genetic drift rather than selection. Even more dramatic are cases of clonal attrition leading to spontaneous regression of CLL, which may reflect effects of genetic drift or negative selection resulting from an interplay of cell-intrinsic and extrinsic factors from the microenvironment (Kwok et al. 2020).
In keeping with these observations, MRD demonstrates a more indolent growth pattern in CLL. Whereas MRD positivity in ALL often portends rapid disease relapse, this relationship is less clear in CLL where other prognostic factors still hold sway. While MRD positivity is associated with higher rates of progression in CLL, MRD status does not always trump the effect of other prognostic biomarkers. For instance, one important biomarker in CLL is IGHV mutation status, with IGHV-mutated status being a well-established positive prognostic biomarker compared to IGHV-unmutated status. In one study, over one third of MRD-positive patients with mutated IGHV remained free from disease progression at thirteen years (Kwok et al. 2016). In contrast, in the same cohort, only sixteen percent of MRD-negative patients with unmutated IGHV were free from disease progression at the same time point.
These examples illustrate how, in CLL, MRD status underdetermines more complex dynamics, giving reasons to doubt that C2 holds in other cancers where MRD has been proposed as a predictive biomarker. Simply assuming that residual cells constituting MRD will be subject to strong selection in a manner sufficient to cause disease relapse is unwarranted given evidence that other processes such as genetic drift can dominate at low population sizes. Unlike selection, such processes may not always tend towards rapid population growth, but rather can result in more indolent growth patterns or even spontaneous clonal extinction in some cases. One must, therefore, exercise caution when intervening on MRD given the risk of overtreating residual cells not destined to cause disease relapse. Moreover, in some cases, intervening may even exacerbate the situation by disrupting stable population dynamics and clonal competition that maintain more indolent growth patterns and selecting for clones that exhibit more rapid expansion (Gruber et al. 2019).
Taken together, C1 and C2 are jointly necessary conditions for the truth of P1. For MRD to imply a high probability of cancer relapse, the cells constituting MRD must not only have the capacity to recapitulate cancer (C1) but they must be subject to strong selection in order to cause relapse (C2). Having called into question the assumptions underlying P1, I now turn to P2.
Risk of relapse is not always an indication for treatment
Recall that P2 in the PBA states that a high probability of cancer relapse above a certain threshold, P(R) > θ, is an indication for treatment T. The truth of P2 is underwritten by a third condition:
Condition 3: A targeted treatment T is capable of reducing the relative fitness of the residual clone constituting MRD compared to normal cells with acceptable toxicity.
Condition 3: targeted intervention
Condition 3 (C3) has to do with the possibility of a targeted treatment effectively reducing the risk of cancer relapse. This moves from thinking about the biological conditions needed for MRD to cause disease relapse to stipulate the clinical conditions required for effective intervention on MRD a means of preventing relapse.
For the purposes of C3, a targeted treatment can be broadly conceptualized. Paradigmatic examples are small molecules, such as imatinib used in chronic myeloid leukemia, or monoclonal antibodies, such as blinatumomab used to treat MRD positivity in ALL. Other approaches, however, can also be considered targeted treatments if they effectively reduce the relative fitness of residual cancer cells. Such approaches might include intensification of chemotherapy or stem cell transplantation, both strategies used for MRD eradication in acute leukemia.
The concept of ‘acceptable toxicity’ also requires clarification. Use of terms such as ‘acceptable’ or ‘tolerable’ to describe toxicity of cancer treatment is problematic due in part to their vagueness (Chin-Yee et al. 2022). Here, I take ‘acceptable’ to mean that an intervention has a risk–benefit profile that most patients would accept based on evidence from clinical outcomes, which includes patient reported outcomes of treatment toxicity.
C3 is demanding, requiring evaluation of clinical outcomes with use of particular therapeutic interventions on MRD. As a result, this premise has been established in fewer cases. The prime example remains ALL, where, as already discussed, MRD positivity can be effectively intervened upon by means of targeted drugs or intensification of treatment. In the case of childhood ALL specifically, while treatment aimed at MRD eradication still causes significant toxicities, in general, patients and clinicians judge the risk–benefit trade-offs to be acceptable given the aggressive nature of the disease and the fact that most children can tolerate intensification of treatment. (This, however, is often not the case for elderly patients with ALL.) Another example is chronic myeloid leukemia where it is possible to effectively prevent clinical relapse by intervening on very low levels of a malignant clone using targeted therapies. Strategies for MRD eradication are currently being studied in other types of acute leukemia, with trials investigating whether early intervention with various drugs can effectively prevent disease relapse (Schuurhuis et al. 2018).
Establishing C3 in different cancers is a key focus of current research in oncology. If established for a given treatment T, it would make true the premise that T is indicated in cases of high probability of cancer relapse as a means of preventing this outcome (P2), thus supporting use of MRD as a predictive biomarker.
Challenges to condition 3
Evidence for C3, however, is more limited in other cancers, perhaps owing to a lack of targeted treatments or differences in risk–benefit trade-offs. Consider the example of multiple myeloma, a more slow-growing cancer of plasma cells which primarily affects older patients and has a well-characterized pre-malignant phase. MRD has been shown to be an important prognostic factor in myeloma, with MRD negativity correlating with improvements in progression free and overall survival across several studies (Munshi et al. 2020; Avet-Loiseau et al. 2020; Cavo et al. 2022). These findings have led to use of MRD as a surrogate endpoint in clinical trials in order to expedite drug approval (FDA 2020). Given MRD’s ability to predict long-term survival in myeloma, many have also suggested it be used as a predictive biomarker—that is, to make MRD negativity the goal of treatment and intervene in cases of MRD positivity to achieve ‘benefit from the deeper response’ (Wallington-Beddoe and Mynott 2021). Several ongoing trials are investigating ‘MRD-driven’ treatment strategies in myeloma (Quach 2022). Whether such strategies prove effective is an empirical question best answered through evidence from well-designed trials with clinically relevant endpoints. Unfortunately, such evidence may remain elusive, as many trials of MRD-directed treatment are limited by their use of surrogate endpoints such as progression free survival or, more often, MRD negativity itself.Footnote 10
Other evidence from myeloma treatment, however, suggests reasons to question the effectiveness of such strategies, given what is known about the risk–benefit trade-offs of more aggressive therapies in patients with this disease. For example, intensive frontline treatment of myeloma with autologous stem cell transplantation, undertaken with the rationale of achieving ‘deep’ responses, has been shown to delay time to disease progression without improving overall survival and at a cost of increased toxicity (Attal et al. 2017; Richardson et al. 2022).Footnote 11 It is well known that in treating older patients with more indolent cancers the benefits of delaying disease progression are often outweighed by both short- and long-term toxicities of more intensive therapies; this is often the case in myeloma, whose therapies are associated with a range of toxicities including secondary malignancies that can accrue prior to disease relapse (Poh et al. 2021). A similar situation might apply in CLL, raising concerns over attempts to eradicate MRD through more intensive treatment strategies as some have advocated (Varghese et al. 2010). There is an analogy here with interventions aimed at disease eradication in more indolent solid tumors, such as low-grade prostate cancers, where risks of aggressive treatments often outweigh benefits.
In short, while attaining MRD negativity is increasingly identified as a goal of treatment in several cancers as a means of preventing cancer relapse, one must carefully consider the disease- and treatment-specific risk–benefit trade-offs before pursuing such strategies. C3 remains to be established in most cancers that aim to use MRD in this capacity. Failure to establish C3—that is, failure to show that a targeted treatment can effectively reduce the risk of relapse posed by MRD and do so with acceptable toxicity—undermines the truth of P2: if C3 does not hold, treatment T is not indicated as an effective strategy to prevent cancer relapse. The PBA, along with the conditions required for each premise and challenges to each condition are summarized in Table 1.
Conclusion
This paper has critically analyzed the expanding use of MRD in oncology and highlighted reasons to doubt its general utility as a predictive biomarker across hematologic cancers. I outlined the argument for MRD as a predictive biomarker, articulating the premises and conditions required for MRD’s success. Specifically, I argued C1-C3 form a set of necessary conditions for MRD’s effective use as a predictive biomarker. While these conditions underwrite MRD’s success in diseases such as ALL, I showed that they are less likely to hold in other cancers where MRD is currently being applied. Challenges to C1-C3 should lead us to temper claims about MRD’s widespread clinical utility. These challenges substantially weaken the strength of the PBA and should make us skeptical that MRD will serve as an effective predictive biomarker across cancer medicine.
Importantly, this paper should not be read as an indictment of the utility of MRD as a biomarker tout court. The ability to detect minute levels of malignant cells represents a significant technical feat. Cancer medicine has come a long way since Farber (1948) first noted ‘no leukemic cells’ in the bone marrows of patients following treatment.
While this paper casts doubt on the broad utility of MRD in cancer, in outlining the PBA it also establishes under what conditions MRD could serve as an effective predictive biomarker. To reiterate, C1-C3 are still empirical conditions—facts about cancer and its treatment that may or may not hold in particular cases. The truth or falsity of C3, for instance, rests on the existence of targeted treatments that enable effective intervention on MRD, best addressed through well-designed clinical trials of MRD-directed treatment that measure patient-centred outcomes.
While clinical trials are no doubt indispensable, to avoid pursuing misguided trials of MRD-directed treatment, attention should also focus on establishing C1 and C2, which provide the biological rationale for MRD’s role as a predictive biomarker. Such a stance aligns with approaches to medical epistemology that emphasize the need to integrate mechanistic rationale with other forms of evidence in medical research (e.g., Clarke et al. 2014; Parkkinen et al. 2018). Here, the PBA and its conditions can serve as useful guides, helping direct inquiry to generate relevant forms of evidence. In this way, my approach is not only critical but also constructive, refocusing attention on the core premises and conditions required for MRD’s success.
More generally, this paper highlights the value of philosophical analysis for examining concepts employed in precision oncology. The rapid pace of translational research in this field creates a pressing need to scrutinize assumptions and clarify rationale to avoid potential harms from misapplication of concepts in practice. Further research is needed to examine issues related to use MRD as a surrogate endpoint in clinical trials, as well as problems of how biomarkers are applied in clinical decision-making.
MRD may hold promise for improving the care of patients with certain cancers. But fulfilling this promise requires that we better understand of the meaning of this measure by critically examining its core premises and conditions for effective use. The arguments in this paper are intended to contribute to this understanding, put forward in the spirit of philosophy ‘in’ science (Pradeu et al. 2024).
Notes
This paper focuses on hematologic cancers, where the concept of MRD and technologies for its measurement are best developed and routinely applied in both research and practice. The concept is sometimes applied to solid tumours (e.g., Mordant et al. 2012), which I do not discuss in this paper.
Residual disease was later posited to persist not only in the bone marrow, but also in so-called ‘sanctuary sites’ not penetrated by many drugs, such as the central nervous system, leading to the incorporation of central nervous system-directed therapy (both chemotherapy and radiation therapy) to prevent disease relapse.
See also Baines et al. (2023) who found that MRD data was submitted in nearly a third of drug applications to the FDA in hematologic cancers and was included in prescribing information in the majority of cases.
For the purposes of this argument, the threshold θ can be understood as a probability of cancer relapse judged sufficiently high to warrant intervention to prevent this outcome.
This, of course, depends on extrinsic factors being able to regenerate CSCs in a clinically relevant time frame so as to cause cancer relapse.
For further discussion and examples of stemness as a relational or systemic property in cancer, see Laplane (2016, 157–78).
For discussion of surrogate endpoints in hematology-oncology clinical trials, see Bommier et al. (2024).
One caveat is that the increase in relative fitness of the residual, treatment-resistant cancer cells may be limited to the context of ongoing therapy. Treatment-resistant cells may not have a selective advantage compared to other non-resistant cells or normal cells outside this context and may even have a fitness decrement owing to the costs of resistance mechanisms (see, for example, Tzoneva et al. 2018). This phenomenon is exploited by so-called ‘adaptive’ treatment strategies which use, for example, ‘treatment vacations’ to help control resistant disease (Gallaher et al. 2018).
Note that even if these residual cancer cells did not initially have a strong selective advantage, e.g., if they grew back slowly at first or were held in check by the microenvironment or immune system until they acquired further fitness enhancing phenotypes or escape mechanisms, for the presence of these cells to imply a high probability of cancer relapse, they would need to reliably acquire a fitness advantage relative to normal cells. The presence of a selective advantage, though it may depend on the temporal frame, is therefore ultimately required for residual cells to imply a high probability of cancer relapse.
Several critics argue that disease progression by itself is not a sufficient endpoint, maintaining that the only clinically meaningful endpoints measure survival or quality of life (see, for discussion, Booth and Eisenhauer 2012; Prasad 2020, pp. 23–36). Others argue that progression free survival remains an intrinsically valuable endpoint in trials.
Older data suggested a potential survival advantage with autologous stem cell transplantation (e.g., Child et al. 2003); however, contemporary trials using newer treatment regimens have not observed this survival advantage (e.g., Attal et al. 2017; Richardson et al. 2022). Upfront stem cell transplantation in myeloma remains a standard treatment approach for eligible patients; however, based on these recent data most guidelines state that delaying transplant is also a reasonable approach.
References
Attal M, Lauwers-Cances V, Hulin C et al (2017) Lenalidomide, bortezomib, and dexamethasone with transplantation for myeloma. N Engl J Med 376(14):1311–1320
Aur RJA, Simone J, Hustu O et al (1971) Central nervous system therapy and combination chemotherapy of childhood lymphocytic leukemia. Blood 37(3):272–281
Avet-Loiseau H, Ludwig H, Landgren O et al (2020) Minimal residual disease status as a surrogate endpoint for progression-free survival in newly diagnosed multiple myeloma studies: a meta-analysis. Clin Lymphoma Myeloma Leuk 20(1):e30–e37
Baines AC, Yazdy MS, Kasamon YL et al (2023) Minimal residual disease data in hematologic malignancy drug applications and labeling: an FDA perspective. Clin Cancer Res 29(15):2748–2752
Batlle E, Clevers H (2017) Cancer stem cells revisited. Nat Med 23(10):1124–1134
Berry DA, Zhou S, Higley H et al (2017) Association of minimal residual disease with clinical outcome in pediatric and adult acute lymphoblastic leukemia: a meta-analysis. JAMA Oncol 3(7):e170580
Bertilaccio MT, Scielzo C, Simonetti G et al (2013) Xenograft models of chronic lymphocytic leukemia: problems, pitfalls and future directions. Leukemia 27(3):534–540
Bommier C, Maurer MJ, Lambert J (2024) What clinicians should know about surrogate endpoints in hematologic malignancies. Blood 144(1):11–20
Booth CM, Eisenhauer EA (2012) Progression-free survival: meaningful or simply measurable. J Clin Oncol 30(10):1030–1033
Bradstock KF, Janossy G, Tidman N et al (1981) Immunological monitoring of residual disease in treated thymic acute lymphoblastic leukaemia. Leuk Res 5(4–5):301–309
Brown PA, Shah B, Advani A et al (2022) Acute lymphoblastic leukemia, version 1.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw 19(9):1079–1089
Brüggemann M, Raff T, Flohr T et al (2006) Clinical significance of minimal residual disease quantification in adult patients with standard-risk acute lymphoblastic leukemia. Blood 107(3):1116–1123
Burger JA, Gribben JG (2014) The microenvironment in chronic lymphocytic leukemia (CLL) and other B cell malignancies: insight into disease biology and new targeted therapies. Semin Cancer Biol 24:71–81
Campana D (1994) Monitoring minimal residual disease in acute leukemia: expectations, possibilities and initial clinical results. Int J Clin Lab Res 24(3):132–138
Campana D, Coustan-Smith E, Janossy G (1990) The immunologic detection of minimal residual disease in acute leukemia. Blood 76(1):163–171
Caravagna G, Heide T, Williams MJ et al (2020) Subclonal reconstruction of tumors by using machine learning and population genetics. Nat Genet 52(9):898–907
Cartwright N (2010) What are randomised controlled trials good for? Philos Stud 147:59–70
Cavo M, San-Miguel J, Usmani SZ et al (2022) Prognostic value of minimal residual disease negativity in myeloma: combined analysis of POLLUX, CASTOR, ALCYONE, and MAIA. Blood 139(6):835–844
Child JA, Morgan GJ, Davies FE et al (2003) High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med 348:1875–1883
Chin-Yee B, Mohammed T, Skorupski C et al (2022) Toxicity reporting is inconsistent and incomplete, and subjective minimizing language is common in acute leukemia clinical trials: a systematic review of randomized controlled trials presented at ASH between 2017–2021. Blood 140(Supplement 1):317–318
Chin-Yee, B (2024) Cancer medicine and precision oncology. In: Schramme T, Walker M (eds) Handbook of the philosophy of medicine. Springer, Dordrecht
Clarke B, Gillies D, Illari P, Russo F, Williamson J (2014) Mechanisms and the evidence hierarchy. Topoi 33:339–360
Clevers H (2011) The cancer stem cell: premises, promises and challenges. Nat Med 17(3):313–319
Dick JE (2008) Stem cell concepts renew cancer research. Blood 112(13):4793–4807
Farber S (1949) Some observations on the effect of folic acid antagonists on acute leukemia and other forms of incurable cancer. Blood 4(2):160–167
Farber S, Diamond LK, Mercer RD, Sylvester RF, Wolff JA (1948) Temporary remissions in acute leukemia in children produced by folic acid antagonist, 4-aminopteroyl-glutamic acid (aminopterin). N Engl J Med 238(23):787–793
FDA (2020) Hematologic malignancies: regulatory considerations for use of minimal residual disease in development of drug and biological products for treatment. Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/hematologic-malignancies-regulatory-considerations-use-minimal-residual-disease-development-drug-and
FDA-NIH Biomarker Working Group (2016) BEST (Biomarkers, endpoints, and other tools) resource. Available at: https://www.ncbi.nlm.nih.gov/books/NBK326791/
Gallaher JA, Enriquez-Navas PM, Luddy KA, Gatenby RA, Anderson ARA (2018) Spatial heterogeneity and evolutionary dynamics modulate time to recurrence in continuous and adaptive cancer therapies. Cancer Res 78(8):2127–2139
George P, Hernandez K, Hustu O, Borella L, Holton C et al (1968) A study of “total therapy” of acute lymphocytic leukemia in children. J Pediatr 72(3):399–408
Ghiaur G, Gerber J, Jones RJ (2012) Concise review: cancer stem cells and minimal residual disease. Stem Cells 30(1):89–93
Greaves M, Janossy G (1978) Patterns of gene expression and the cellular origins of human leukaemias. Biochim Biophys Acta 516(2):193–230
Gruber M, Bozic I, Leshchiner I, Livitz D et al (2019) Growth dynamics in naturally progressing chronic lymphocytic leukaemia. Nature 570(7762):474–479
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144(5):646–674
Kikushige Y, Ishikawa F, Miyamoto T et al (2011) Self-renewing hematopoietic stem cell is the primary target in pathogenesis of human chronic lymphocytic leukemia. Cancer Cell 20(2):246–259
Kukla QR (2022) What counts as a disease, and why does it matter? J Philos Disabil 2:130–156
Kwok M, Rawstron AC, Varghese A et al (2016) Minimal residual disease is an independent predictor for 10-year survival in CLL. Blood 128(24):2770–2773
Kwok M, Oldreive C, Rawstron AC et al (2020) Integrative analysis of spontaneous CLL regression highlights genetic and microenvironmental interdependency in CLL. Blood 135(6):411–428
Kyle RA, Durie BGM, Rajkumar SV et al (2010) Monoclonal gammopathy of undetermined significance (MGUS) and smoldering (asymptomatic) multiple myeloma: IMWG consensus perspectives risk factors for progression and guidelines for monitoring and management. Leukemia 24(6):1121–1127
Lapidot T, Sirard C, Vormoor J et al (1994) A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature 367(6464):645–648
Laplane L (2016) Cancer stem cells. Harvard University Press
Laplane L (2018) Cancer stem cells modulate patterns and processes of evolution in cancers. Biol Philos 33:18
Lyne A-M, Laplane L, Perié L (2021) To portray clonal evolution in blood cancer, count your stem cells. Blood 137(14):1862–1870
Merlo LM, Pepper JW, Reid BJ, Maley CC (2006) Cancer as an evolutionary and ecological process. Nat Rev Cancer 6(12):924–935
Mordant P, Loriot Y, Lahon B et al (2012) Minimal residual disease in solid neoplasia: new frontier or red-herring. Cancer Treat Rev 38:101–110
Munshi NC, Avet-Loiseau H, Anderson KC et al (2020) A large meta-analysis establishes the role of MRD negativity in long-term survival outcomes in patients with multiple myeloma. Blood Adv 4(23):5988–5999
Nowell PC (1976) The clonal evolution of tumor cell populations. Science 194(4260):23–28
Okasha S (2024) Cancer and the levels of selection. Br J Philos Sci 75(3):1–24
Parkkinen V-P, Wallman C, Wilde M et al (2018) Evaluating evidence of mechanisms in medicine. Springer
Plutynski A (2018) Explaining cancer. Oxford University Press, Oxford
Plutynski A (2019) Cancer modeling: the advantages and limitations of multiple perspectives. In: Massimi M, McCoy CD (eds) Understanding perspectivism. Routledge, New York
Plutynski A (2022) Why precision oncology is not very precise (and why this should not surprise us). In: Beneduce C, Bertolaso M (eds) Personalized medicine in the making. Springer, Dordrecht
Poh C, Keegan T, Rosenberg AS (2021) Second primary malignancies in multiple myeloma: a review. Blood Rev 46:100757
Pradeu T, Daignan-Fornier B, Ewald A et al (2023) Reuniting philosophy and science to advance cancer research. Biol Rev 98(5):1668–1686
Pradeu T, Lemoine M, Khelfaoui M, Gingras Y (2024) Philosophy in science: Can philosophers of science permeate through science and produce scientific knowledge. Br J Philos Sci 75(2):375-416
Prasad VK (2020) Malignant: How bad policy and bad evidence harm people with cancer. Johns Hopkins University Press, Baltimore
Pui CH, Evans WE (2013) A 50-Year journey to cure childhood acute lymphoblastic leukemia. Semin Hematol 50(3):185–196
Purroy N, Wu CJ (2017) Coevolution of leukemia and host immune cells in chronic lymphocytic leukemia. Cold Spring Harb Perspect Med 7(4):a026740
Quach H (2022) MRD end point in myeloma: ready for prime time. Blood 139(6):799–802
Raff T, Gökbuget N, Lüschen S et al (2007) Molecular relapse in adult standard-risk ALL patients detected by prospective MRD monitoring during and after maintenance treatment: data from the GMALL 06/99 and 07/03 trials. Blood 109(3):910–915
Rajkumar SV (2008) Treatment of myeloma: cure vs control. Mayo Clin Proc 83(10):1142–1145
Rawstron AC, Bennett FL, O'Connor SJM et al (2008) Monoclonal B-cell lymphocytosis and chronic lymphocytic leukemia. N Engl J Med 359(6):575–583
Richardson PG, Jacobus SJ, Weller EA et al (2022) Triplet therapy, transplantation, and maintenance until progression in myeloma. N Engl J Med 387(2):132–147
Riddell J, Gazit R, Garrison BS et al (2014) Reprogramming committed murine blood cells to induced hematopoietic stem cells with defined factors. Cell 157(3):549–564
Rogers WA, Walker MJ (2017) The line-drawing problem in disease definition. J Med Philos 42(4):405–423
Saygin C, Cannova J, Stock W, Muffly L (2022) Measurable residual disease in acute lymphoblastic leukemia: methods and clinical context in adult patients. Haematologica 107(12):2783–2793
Schuurhuis GJ, Heuser M, Freeman S et al (2018) Minimal/measurable residual disease in AML: a consensus document from the European LeukemiaNet MRD working party. Blood 131(12):1275–1291
Schwartz PH (2007) Defining dysfunction: natural selection, design, and drawing a line. Philos Sci 74(3):364–385
Schwartz PH (2014) Small tumors as risk factors not disease. Philos Sci 81(5):986–998
Shanafelt TD, Wang X, Kay NE et al (2019) Ibrutinib-rituximab or chemoimmunotherapy for chronic lymphocytic leukemia. N Engl J Med 381(5):432–443
Simone JV (1981) Outlook for acute lymphocytic leukemia in children in 1982. Annu Rev Med 32(1):207–212
Simone J, Aur RJA, Hustu O, Pinkel D (1972) Total therapy studies of acute lymphocytic leukemia in children. Current results and prospects for cure. Cancer 30(6):1488–1494
Stow P, Key L, Chen X et al (2010) Clinical significance of low levels of minimal residual disease at the end of remission induction therapy in childhood acute lymphoblastic leukemia. Blood 115(23):4657–4663
Thompson PA, Wierda WG (2016) Eliminating minimal residual disease as a therapeutic end point: working toward cure for patients with CLL. Blood 127(3):279–286
Tzoneva G, Dieck CL, Oshima K et al (2018) Clonal evolution mechanisms in NT5C2 mutant-relapsed acute lymphoblastic leukaemia. Nature 553(7689):511–514
van Rhenen A, Feller N, Kelder A et al (2005) High stem cell frequency in acute myeloid leukemia at diagnosis predicts high minimal residual disease and poor survival. Clin Cancer Res 11(18):6520–6527
Varghese AM, Rawstron AC, Hillmen P (2010) Eradicating minimal residual disease in chronic lymphocytic leukemia: should this be the goal of treatment. Curr Hematol Malig Rep 5(1):35–44
Wallington-Beddoe CT, Mynott RL (2021) Prognostic and predictive biomarker developments in multiple myeloma. J Hematol Oncol 14(1):151
Wang XV, Hanson C, Tschumper R et al (2021) Measurable residual disease does not preclude prolonged progression-free survival in CLL treated with ibrutinib. Blood 138(26):2810–2827
Watson CJ, Papula AL, Poon GYP et al (2020) The evolutionary dynamics and fitness landscape of clonal hematopoiesis. Science 367(6485):1449–1454
Williams MJ, Werner B, Barnes CP, Graham TA, Sottoriva A (2016) Identification of neutral tumor evolution across cancer types. Nat Genet 48(3):238–244
Acknowledgements
Thanks to Jacob Stegenga, Anya Plutynski, Lucie Laplane, Pierre Sujobert, Adrià Segarra, Ina Jäntgen, Jonathan Fuller, Oliver Holdsworth, Hamed Tabatabaei Ghomi, Ian Chin-Yee, Anargyros Xenocostas, Mike Keeney, Ben Hedley, Lori Lowes, Alla Iansavitchene, and two reviewers at Biology & Philosophy, as well as audiences at Cambridge, Bordeaux, and Toronto for helpful feedback and discussion.
Funding
The author received funding from the Gates Cambridge Trust and the Social Sciences and Humanities Research Council of Canada.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author has no competing interests to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chin-Yee, B. Minimal residual disease: premises before promises. Biol Philos 39, 17 (2024). https://doi.org/10.1007/s10539-024-09958-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10539-024-09958-w