Abstract
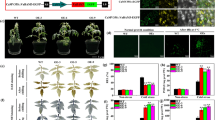
In chloroplasts and mitochondria, antioxidant mechanisms include the ascorbate-glutathione cycle, and monodehydroascorbate reductase (MDHAR) is important for regeneration of ascorbate (AsA) from monodehydroascorbate (MDHA). To improve detoxification of reactive oxygen species (ROS), we established a construct of the MDHAR gene from Brassica rapa fused to the targeting signal peptides of Pisum sativum glutathione reductase (GR), which was controlled by a stress-inducible SWPA2 promoter, and introduced this expression system into Arabidopsis thaliana. Transgenic (TG) plants overexpressing BrMDHAR targeted to chloroplasts and mitochondria through signal peptides showed an elevated MDHAR activity and an increased ratio of AsA to dehydroascorbate (DHA) when compared to wild-type (WT) plants under a freezing stress. These led to increased photosynthetic parameters, redox homeostasis, and biomass in TG plants when compared to the WT plants. Our results suggest that the overexpression of the BrMDHAR gene targeted to chloroplasts and mitochondria conferred an enhanced tolerance against the freezing stress, and a stress adaptation of dual-targeted BrMDHAR was better than that of single BrMDHAR.
Similar content being viewed by others
Abbreviations
- APX:
-
ascorbate peroxidase
- AsA:
-
ascorbate
- BrMHAR :
-
Brassica rapa monodehydroascorbate reductase gene
- DCFH-DA:
-
6-carboxy-2′,7′-dichlorofluorescin diacetate
- DHA:
-
dehydroascorbate
- DHAR:
-
dehydroascorbate reductase
- GPX:
-
glutathione peroxidase
- GR:
-
glutathione reductase
- GSH:
-
glutathione
- MDA:
-
malondialdehyde
- MDHA:
-
monodehydroascorbate
- MDHAR:
-
monodehydroascorbate reductase
- Prx Q:
-
peroxiredoxin Q
- ROS:
-
reactive oxygen species
- SOD:
-
superoxide dismutase
- SWPA2 promoter:
-
stress-inducible promoter from sweet potato ascorbate peroxidase
- TBA:
-
thiobarbituric acid
- TCA:
-
trichloroacetic acid
- TG:
-
transgenic
- WT:
-
wild type
References
Asada, K.: The water-water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. — Annu. Rev. Plant Physiol. Plant mol. Biol. 50: 601–639, 1999.
Asada, K., Takahashi M.: Production and scavenging of active oxygen in photosynthesis. — In: Kyle, D.J., Osmond, C.B., Arntzen, C.J. (ed.): Photoinhibition. Pp 227–287. Elsevier Science Publishers, Amsterdam 1987.
Carrie, C., Giraud, E., Duncan, O., Xu, L., Wang, Y., Huang, S., Clifton, R., Murcha, M., Filipovska, A., Rackham, O., Vrielink, A., Whelan, J.: Conserved and novel functions for Arabidopsis thaliana MIA40 in assembly of proteins in mitochondria and peroxisomes. — J. biol. Chem. 285: 36138–36148, 2010.
Carrie, C., Giraud, E., Whelan, J.: Protein transport in organelles: dual tatgeting of proteins to mitochondria and chloroplasts. — FEBS J. 276: 1187–1195, 2009a.
Carrie, C., Kűhn, K., Murcha, M.W., Duncan, O., Small, I.D., O’Toole, N., Whelan, J.: Approaches to defining dualtargeted proteins in Arabidopsis. — Plant J. 57: 1128–1139, 2009b.
Carrie, C., Small, I.: A re-evaluation of dual-targeting of proteins to mitochondria and chloroplasts. — Biochim. biophys. Acta 1833: 253–259, 2013.
Carrie, C., Whelan, J.: Widespread dual targeting of proteins in land plants: when, where, how and why. — Plant Signal. Behav. 8(Suppl.): e25034, 2013.
Chacinska, A., Pfannschmidt, S., Wiedemann, N., Kozjak, V., Sanjuán Szklarz, L.K., Schulze-Specking, A., Truscott, K.N., Guiard, B., Meisinger, C., Pfanner, N.: Essential role of Mia40 in import and assembly of mitochondrial intermembrane space proteins. — EMBO J. 23: 253–259, 2004.
Chen, H., Nelson, R.S., Sherwood, J.L.: Enhanced recovery of transformants of Agrobacterium tumefaciens after freezethaw transformation and drug selection. — Biotechniques 16: 664–668, 1994.
Chew, O., Rudhe, C., Glaser, E., Whelan, J.: Characterization of the targeting signal of dual-targeted pea glutathione reductase. — Plant mol. Biol. 53: 341–356, 2003a.
Chew, O., Whelan, J., Millar, A.H.: Molecular definition of the ascorbate-glutathione cycle in Arabidopsis mitochondria reveals dual-targeting of antioxidant defenses in plants. — J. biol. Chem. 278: 46869–46877, 2003b.
Clough, S., Bent, A.: Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. — Plant J. 16: 735–743, 1998.
Creissen, G., Edwards, E.A., Enard, C., Wellburn, A., Mullineaux, P.: Molecular characterization of glutathione reductase cDNAs from pea (Pisum sativum L.). — Plant J. 2: 129–131, 1992.
Creissen, G., Reynolds, H., Xue, Y., Mullineaux, P.: Simultaneous targeting of pea glutathione reductase and a bacterial fusion protein to chloroplasts and mitochondtria in transgenic tobacco. — Plant J. 8: 167–165, 1995.
Dalton, D., Baird, L.M., Langeberg, L., Tauqher, C.Y., Anyan, W.R., Vance, C.P., Sarath, G.: Subcellular localization of oxygen defense enzymes in soybean (Glycine max [L.] Merr.) root nodules. — Plant Physiol. 102: 481–489, 1993.
Dhindsa, R.S., Plumb-Dhindsa, P., Thorpe, T.A.: Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. — J. exp. Bot. 32: 93–101, 1981.
Eubel, H., Heazlewood, J.L., Millar, A.H.: Isolation and subfractionation of plant mitochondria for proteomic analysis. — Methods mol. Biol. 355: 49–62, 2006.
Foreman, J., Demidchik, V., Bothwell, J.H., Mylona, P., Miedema, H., Torres, M.A., Linstead, P., Costa, S., Brownlee, C., Jones, J.D., Davies, J.M., Dolan. L.: Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. — Nature 422: 442–446, 2003.
Foyer, C.H., Halliwell, B.: The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. — Planta 133: 21–25, 1976.
Fridovich, I.: Biological effects of the superoxide radical. — Arch. Biochem. Biophys. 247: 1–11, 1986.
Gillespie, K., Ainsworth, E.: Measurement of reduced, oxidized and total ascorbate content in plants. — Nat. Protocols 2: 871–874, 2007.
Halliwell, B.: Oxidative stress and neurodegeneration: where are we now? — J. Neurochem. 97: 1634–1658, 2006.
Halliwell, B., Gutteridge, J.M.: Role of free radicals and catalytic metal ions in human disease: an overview. — Methods Enzymol. 186: 1–85, 1990.
Harlow, E., Lane, D.: Antibodies: A Laboratory Manual. — Cold Spring Harbor Press, New York 1988.
Heath, R., Packer, L.: Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. — Arch. Biochem. Biophys. 125: 189–198, 1968.
Hossain, M., Nakano, Y., Asada, K.: Monodehydroascorbate reductase in spinach chloroplasts and its participation in regeneration of ascorbate for scavenging hydrogen peroxide. — Plant Cell Physiol. 25: 385–395, 1984.
Ishikawa, T., Takeda, T., Shigeoka, S., Hirayama, O., Mitsunaga, T.: Hydrogen peroxide generation in organelles of Euglena gracilis. — Phytochemistry 33: 1297–1299, 1993.
Jansson, S.: The light-harvesting chlorophyll a/b-binding proteins. — Biochim. biophys. Acta 1184: 1–19, 1994.
Jezek, P., Hlavatá, L.: Mitochondria in homeostasis of reactive oxygen species in cell, tissues, and organism. — Int. J. Biochem. Cell Biol. 37: 2478–2503, 2005.
Jimenez, A., Hernandez, J.A., Del Rio, L.A., Sevilla, F.: Evidence for the presence of the ascorbate-glutathione cycle in mitochondria and peroxisomes of pea leaves. — Plant Physiol. 114: 275–284, 1997.
Karimi, M., Inze, D., Depicker, A.: GATEWAY vectors for Agrobacterium-mediated plant transformation. — Trends Plant Sci. 7: 193–195, 2002.
Kim, K.Y., Kwon, S.Y., Lee, H.S., Hur, Y., Bang, J.W., Kwak, S.S.: A novel oxidative stress-inducible peroxidase promoter from sweetpotato: molecular cloning and characterization in transgenic tobacco plants and cultured cells. — Plant mol. Biol. 51: 831–838, 2003.
Levitan, A., Trebitsh, T., Kiss, V., Pereq, Y., Danqoor, I., Danon, A.: Dual targeting of the protein disulfide isomerase RB60 to the chloroplast and the endoplasmic reticulum. — Proc. nat. Acad. Sci. USA 102: 6225–6230, 2005.
Lichtenthaler, H.: Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. — Methods Enzymol. 148: 350–382, 1987.
Mittler, R.: Oxidative stress, antioxidants and stress tolerance. — Trends Plant Sci. 7: 405–410, 2002.
Mittova, V., Tal, M., Volokita, M., Guy, M.: Up-regulation of the leaf mitochondrial and peroxisomal antioxidative systems in response to salt-induced oxidative stress in the wild salt-tolerant tomato species Lycopersicon pennellii. — Plant Cell Environ. 26: 845–856, 2003.
Mizuno, M., Kamei, M., Tsuchida, H.: Ascorbate peroxidase and catalase cooperate for protection against hydrogen peroxide generated in potato tubers during low-temperature storage. — Biochem. mol. Biol. Int. 44: 717–726, 1998.
Møller, I.M.: Plant mitochondria and oxidative stress: electron transport, NADPH turnover, and metabolism of reactive oxygen species. — Annu. Rev. Plant Physiol. Plant mol. Biol. 52: 561–591, 2001.
Moon, H., Lee, B., Choi, G., Shin, D., Prasad, D.T., Lee, O., Kwak, S.S., Kim, D.H., Nam, J., Bahk, J., Hong, J.C., Lee, S.Y., Cho, M.J., Lim, C.O., Yun, D.J.: NDP kinase 2 interacts with two oxidative stress-activated MAPKs to regulate cellular redox state and enhances multiple stress tolerance in transgenic plants. — Proc. nat. Acad. Sci. USA 100: 358–363, 2003.
Morgante, C.V., Rodrigues, R.A., Marbach, P.A., Borgonovi, C.M., Moura, D.S., Silva-Filho, M.C.: Conservation of dual-targeted proteins in Arabidopsis and rice points to a similar pattern of gene-family evolution. — Mol. Genet. Genomics 281: 525–538, 2009.
Navrot, N., Rouhier, N., Gelhaye, E., Jacquot, J.: Reactive oxygen species generation and antioxidant systems in plant mitochondria. — Physiol. Plant. 129: 185–195, 2007.
Njus, D., Kelley, P.M.: Vitamins C and E donate single hydrogen atoms in vivo. — FEBS Lett. 284: 147–151, 1991.
Noctor, G., Foyer, C.: Ascorbate and glutathione: keeping active oxygen under control. — Annu. Rev. Plant Physiol. Plant mol.Biol. 49: 249–279, 1998.
Obara, K., Sumi, K., Fukuda, H.: The use of multiple transcription starts causes the dual targeting of Arabidopsis putative monodehydroascorbate reductase to both mitochondria and chloroplasts. — Plant Cell physiol. 43: 697–705, 2002.
Prasad, T.K., Anderson, M.D., Martin, B.A., Stewart, C.R..: Evidence for chilling-induced oxidative stress in maize seedlings and a regulatory role for hydrogen peroxide. — Plant Cell 6: 65–74, 1994.
Puntarulo, S., Galleano, M., Sanchez, R.A., Boveris, A.: Superoxide anion and hydrogen peroxide metabolism in soybean embryonic axes during germination. — Biochim. biophys. Acta 1074: 277–283, 1991.
Reumann, S., Babujee, L., Ma, C., Wienkoop, S., Siemsen, T., Antonicelli, G.E., Rasche, N., Lűder, F., Weckwerth, W., Jahn, O.: Proteome analysis of Arabidopsis leaf peroxisomes reveals novel targeting peptides, metabolic pathways, and defense mechanisms. — Plant Cell 19: 3170–3193, 2007.
Rokov-Plavec, J., Dulic, M., Duchêne, A.M., Weygand-Durasevic, I.: Dual targeting of organellar seryl-tRNA synthetase to maize mitochondria and chloroplasts. — Plant Cell Rep. 27: 1157–1168, 2008.
Rudhe, C., Clifton, R., Whelan, J., Glaser, E.: N-terminal domain of the dual-targeted pea glutathione reductase signal peptide controls organellar targeting efficiency. — J. mol. Biol. 324: 577–585, 2002.
Sapir-Mir, M., Mett, A., Belausov, E., Tal-Meshulam, S., Frydman, A., Gidoni, D., Eyal, Y.: Peroxisomal localization of Arabidopsis isopentenyl diphosphate isomerases suggests that part of the plant isoprenoid mevalonic acid pathway is compartmentalized to peroxisomes. — Plant Physiol. 148: 1219–1228, 2008.
Schroeder, J.I., Allen, G.J., Hugouvieux, V., Kwak, J.M., Waner, D.: Guard cell signal transduction. — Annu. Rev. Plant Physiol. Plant mol. Biol. 52: 627–658, 2001a.
Schroeder, J.I., Kwak, J.M., Allen, G.J.: Guard cell abscisic acid signalling and engineering drought hardiness in plants. — Nature 410: 327–330, 2001b.
Schwacke, R., Fischer, K., Ketelsen, B., Krupinska, K., Krause, K.: Comparative survey of plastid and mitochondrial targeting properties of transcription factors in Arabidopsis and rice. — Mol. Genet. Genomics 277: 631–646, 2007.
Scrutton, N.S., Berry, A., Perham, R.N.: Redesign of the coenzyme specificity of a dehydrogenase by protein engineering. — Nature 343: 38–43, 1990.
Shultz, R., Settlage, S.B., Hanley-Bowdoin, L., Thompson, W.F.: A trichloroacetic acid-acetone method greatly reduces infrared autofluorescence of protein extracts from plant tissue. — Plant mol. Biol. Rep. 23: 405–409, 2005.
Spickett, C.M., Smirnoff, N., Pitt, A.R.: The biosynthesis of erythroascorbate in Saccharomyces cerevisiae and its role as an antioxidant. — Free Radicals Biol. Med. 28: 183–192, 2000.
Sweetlove, L., Heazlewood, J.L., Herald, V., Holtzapffel, R., Day, D.A., Leaver, C.J., Milliar, A.H.: The impact of oxidative stress on Arabidopsis mitochondria. — Plant J. 32: 891–904, 2002.
Wagner, A.M.: A role for active oxygen species as second messengers in the induction of alternative oxidase gene expression in Petunia hybrida cells. — FEBS Lett. 368: 339–342, 1995.
Wang, H., Joseph, J.: Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. — Free Radicals Biol. Med. 27: 612–616, 1999.
Wolff, S.: Ferrous ion oxidation in presence of ferric ion indicator xylenol orange for measurement of hydroperoxides. — Methods Enzymol. 233: 182–189, 1994.
Xu, L., Carrie, C., Law, S.R., Murcha, M.W., Whelan, J.: Acquisition, conservation, and loss of dual-targeted proteins in land plants. — Plant Physiol. 161: 644–662, 2013.
Yoo, S.D., Cho, Y.H., Sheen, J.: Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. — Nat. Protoc. 2: 1565–1572, 2007.
Author information
Authors and Affiliations
Corresponding author
Additional information
Acknowledgements: This work was supported by a grant from the Next-Generation BioGreen 21 Program (No. PJ008060), Rural Development Administration, South Korea. I.S. Kim and H.S. Yoon equally contributed to this study.
Rights and permissions
About this article
Cite this article
Shin, S.Y., Kim, Y.S., Kim, I.S. et al. The expression of BrMDHAR gene in chloroplasts and mitochondria enhances tolerance to freezing stress in Arabidopsis thaliana . Biol Plant 58, 456–468 (2014). https://doi.org/10.1007/s10535-014-0416-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10535-014-0416-7