Abstract
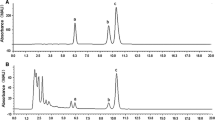
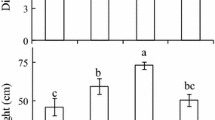
Photosynthetic parameters were measured in two invasive weeds, Mikania micrantha and Chromolaena odorata, grown in soil under full, medium, and low irradiance and full, medium, and low water supply. Both species showed significantly higher net photosynthetic rate, quantum yield of PS 2 photochemistry and photochemical quenching coefficient under high than low irradiance. For M. micrantha, low irradiance caused decreased chlorophyll content (Chl), Chl a/b ratio and maximum photochemical efficiency of PS 2 (Fv/Fm), while drought decreased Chl content and Fv/Fm and increased nonphotochemical quenching (NPQ). However, these parameters were much less affected in C. odorata except that Chl content and NPQ slightly increased under drought and high irradiance. High irradiance increased xanthophyll pools in both species, especially M. micrantha under combination with drought.
Similar content being viewed by others
Abbreviations
- A:
-
antheraxanthin
- FC:
-
field water capacity
- FI:
-
full irradiance
- Fv/Fm :
-
maximum photochemical efficiency of PS 2
- FW:
-
full water
- LI:
-
low irradiance
- LW:
-
low water
- MI:
-
medium irradiance
- MW:
-
medium water
- NPQ:
-
nonphotochemical quenching coefficient
- PN :
-
net photosynthetic rate
- qP:
-
photochemical quenching coefficient
- V:
-
violaxanthin
- Z:
-
zeaxanthin
- ΦPS2 :
-
quantum yield of PS 2 photochemistry
References
Adams, W.W. III., Demmig-Adams, B., Logan, B.A., Baker, D.H., Osmond, C.B.: Rapid changes in xanthophyll cycle-dependent energy dissipation and photosystem II efficiency in two vines, Stephania japonica and Smilas australis, growing in the understory of an open Eucalyptus forest. — Plant Cell Environ. 22: 125–136, 1999.
Chow, W.S.: Photoprotection and photoinhibition. — In: Bittar, E.E., Barber, J. (ed.): Advances in Molecular and Cell Biology: Molecular Process of Photosynthesis. Vol. X. Pp. 151–196. JAI Press, Greenwich 1994.
Demmig-Adams, B., Adams, W.W., III.: Photoprotection and other responses of plants to high light stress. — Annu. Rev. Plant Physiol. Plant mol. Biol. 43: 599–626, 1992.
Giardi, M.T., Cona, A., Geiken, B., Kucera, T., Masojidek, J., Mattoo, A.K.: Long-term drought stress induces structural and functional reorganization of photosystem II. — Planta 199: 118–125, 1996.
Gilmore, A.M.: Mechanistic aspects of xanthophyll cycle-dependant photoprotection in higher plant chloroplast and leaves. — Physiol. Plant. 99: 118–125, 1997.
Gilmore, A.M., Yamamoto, H.Y.: Resolution of lutein and zeaxanthin using a nonendcapped, lightly carbon-loaded C-18 high-performance liquid chromatographic column. — J. Chromatography 543: 137–145, 1991.
Gimenez, C., Mitchell, V.J., Lawlor, D.W.: Regulation of photosynthetic rate of two sunflower hybrids under water stress. — Plant Physiol. 96: 635–643, 1992.
Golan, T., Müller-Moulé, P., Niyogi, K.K.: Photoprotection mutants of Arabidopsis thaliana acclimate to high light by increasing photosynthesis and specific antioxidants. — Plant Cell Environ. 29: 879–887, 2006.
Holm, L.G., Plucknett, D.L., Pancho, J.V., Herberger, J.P. (ed.): The World’s Worst Weeds: Distribution and Biology. — University Press of Hawaii, Honolulu 1977.
Liao, F.Y., Xie, Y., He, P., Fan, Y.M.: The effect of different light intensity on the growth and photosystem of Mikania micrantha Kunth. — Life Sci Res. 7: 355–359, 2003. [In Chin.]
Lichtenthaler, H.K., Buschmann, C., Döll, M., Fietz, H.J., Bach, T., Kozel, U., Meier, D., Rahmsdorf, U.: Photosynthetic activity, chloroplast ultrastructure, and leaf characteristics of high-light and low-light plants and of sun and shade leaves. — Photosynth Res. 2: 115–141, 1981.
Lin, Z.F., Li, S.S., Lin, G.Z., Sun, G.C., Guo, J.Y.: [Superoxide dismutase activity and liquid peroxidation in relation to senescence of rice leaves]. — Acta bot. sin. 26: 605–615, 1984. [In Chin.]
Lu, C.M., Qiu, N.W., Lu, Q.T., Wang, B.S., Kuang, T.Y.: PSII photochemistry, thermal energy dissipation, and the xanthopyhll cycle in Kalanchoë daigremontiana exposed to a combination of water stress and high light. — Physiol. Plant. 118: 173–182, 2003.
Melgar, J.C., Syvertsen, J.P., Martínez, V., García-Sánchez, F.: Leaf gas exchange, water relations, nutrient content and growth in citrus and olive seedlings under salinity. — Biol. Plant. 52: 385–390, 2008.
Pogson, B., Niyogi, K.K., Björkman, O., Dellapenna, D.: Altered xanthophylls compositions adversely affect chlorophyll accumulation and nonphotochemical quenching in Arabidopsis mutants. — Proc. nat. Acad. Sci. USA. 95: 13324–13329, 1998.
Qiu, N.W., Lu, Q.T., Lu, C.M.: Photosynthesis, photosystem II efficiency and the xanthophyll cycle in the salt-adapted halophyte Atriplex centralasiatica. — New Phytol. 159: 479–486, 2003.
Schiefthaler, U., Russell, A.W., Bolhàr-Nordenkampf, H.R., Critchley, C.: Photoregulation and photodamage in Schefflera arboricola leaves adapted to different light environments. — Aust. J. Plant Physiol. 26: 485–494, 1997.
Souza, R.P., Machado, E.C., Silva, J.A.B., Lagpa, A.M.M.A., Silveira, J.A.G.: Photosynthetic gas exchange, chlorophyll fluorescence and some associated metabolic changes in cowpea (Vigna unguiculata) during water stress and recovery. — Environ. exp. Bot. 51: 45–56, 2004.
Van Kooten, O., Snel, J.F.H.: The use of chlorophyll fluorescence nomenclature in plant stress physiology. — Photosynth. Res. 25: 147–150, 1990.
Wang, J.F., Feng, Y.L., Li, Z.: Acclimation of photosynthesis to growth light intensity in Chromolaena odorata L. and Gynura sp. — J. Plant Physiol. mol Biol. 29: 542–548, 2003.
Yu, D.J., Kim, S.J., Lee, H.J.: Stomatal and non-stomatal limitations to photosynthesis in field-grown grapevine cultivars. — Biol. Plant. 53: 133–137, 2009.
Zhang, L.L., Wen, D.Z.: Structural and physiological responses of two invasive weeds Mikania micrantha and Chromolaena odorata to contrasting light and soil water conditions. — J. Plant Res. 122: 69–79, 2009.
Author information
Authors and Affiliations
Corresponding author
Additional information
Acknowledgements: We thank Prof. Zhi-Fang Lin, Prof. Han-Hui Kuang, and two anonymous reviewers for critical comments on earlier version of this manuscript. The study was funded by National Natural Science Foundation of China (No. 30630015) and the Pear River Delta Urban Forest and the Environment Research Program of SCBG (200603).
Rights and permissions
About this article
Cite this article
Zhang, L.L., Wen, D.Z. & Fu, S.L. Responses of photosynthetic parameters of Mikania micrantha and Chromolaena odorata to contrasting irradiance and soil moisture. Biol Plant 53, 517–522 (2009). https://doi.org/10.1007/s10535-009-0093-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10535-009-0093-0