Abstract
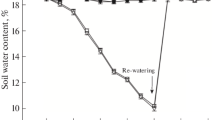
Long-term drought stress on photosystem II (PSII) was studied in pea (Pisum sativum L.) seedlings. Drought stress (reduction of water content by 35–80%) led to a considerable depletion of the PSII core, and the remaining PSII complex appeared to be functional and reorganized, with a unit size (LHCP/PSII core) twofold greater than that of well-irrigated plants. By immunoblotting analysis of the PSII proteins from grana and stroma lamellae, the enhanced degradation of CP43 and D1 proteins was observed in water-stressed plants. Also, water stress caused increased phosphorylation of the PSII core and increased D1 protein synthesis. Water-stress-mediated increase in D1 synthesis did not occur when plants were exposed to photoinhibitory light. The depletion of the PSII core was essentially reversed when water-stressed plants grown at low visible irradiance were watered. We suggest that the syndrome caused by the effect of long-term water stress on photosynthesis is a combination of at least two events: a reduction in the number of active PSII centres caused by a physical destabilization of the PSII core and a PSII reorganization with enhanced D1 turnover to counteract the core depletion.
Similar content being viewed by others
Abbreviations
- Chl:
-
chlorophyll
- CP43 and CP47:
-
β-carotene-Chla-proteins of PSII core
- DCPIP:
-
2,6-dichlorophenolindophenol
- DPC:
-
diphenylcarbazide
- Fv/Fm:
-
the ratio of yield of variable fluorescence to yield of maximal fluorescence when all reaction centres are closed
- LHC(P):
-
light-harvesting complex (proteins)
- Wc:
-
water content
References
Allen JF (1992) Protein phosphorylation in regulation of photosynthesis. Biochim Biophys Acta 1098: 275–335
Anderson JM, Aro EM (1994) Grana stacking and protection of photosystem II in thylakoid membranes of higher plants under sustained high irradiance. Photosynth Res 41: 315–326
Aro EM, Virgin I, Andersson B (1993) Photoinhibition of photosystem II. Inactivation, protein damage and turnover. Biochim Biophys Acta 1143: 113–134
Baker NR (1991) Possible role of photosystem II in environmental perturbations of photosynthesis. Physiol Plant 81: 563–570
Barbato R, Friso G, Giardi MT, Rigoni F, Giacometti GM (1991) Breakdown of the photosystem II reaction center D1 protein under photoinhibitory conditions: identification and localization of the C-terminal degradation product. Biochemistry 30: 10020–10026
Barber J, Andersson B (1992) Too much of a good thing: light can be bad for photosynthesis. TIBS 17: 61–66
Beadle CL, Ludlow MM, Honeysett JL (1993) Water relations. In: Hall, DO et al. (eds) Photosynthesis and production in a changing environment: a field and laboratory manual. Chapman & Hall, London, pp 113–128
Björkmann O, Powles SB (1984) Inhibition of photosynthetic reactions under water stress: interaction with light level. Planta 161: 490–504
Boyer JS, Bowen BL (1970) Inhibition of oxygen evolution in chloroplast isolated from leaves with low water potentials. Plant Physiol 45: 612–615
Bracht E, Trebst A (1994) Hypothesis of the control of D1 turnover by nuclear coded proteins in Chlamydomonas reinhardii. Z Naturforsch 49c: 439–446
Callahan FE, Wergin WP, Nelson N, Edelman M, Mattoo, AK (1989) Distribution of thylakoid proteins between stromal and grana lamellae in Spirodela. Plant Physiol 91: 629–635
Callahan FE, Ghirardi ML, Sopory SK, Mehta AM, Edelman M, Mattoo AK (1990) A novel metabolic form of the 32 kDa-D1 protein in the grana-localized reaction center of photosystem II. J Biol Chem 265: 15357–15360
Chaves MM (1991) Effects of water deficits on carbon assimilation. J Exp Bot 42: 1–16
Cornic G, Ghashghaie J, Genty B, Briantais J-M (1992) Leaf photosynthesis is resistant to a mild drought stress. Photosynthetica 27: 295–309
Critchley C (1988) The molecular mechanism of photoinhibition, facts and fiction. Aust J Plant Physiol 15: 27–41
Critchley C, Russell AW (1994) Photoinhibition of photosynthesis in vivo: the role of protein turnover in photosystem II. Physiol Plant 92: 188–196
Draber W, Tietjen K, Kluth JF, Trebst A (1991) Herbicides in photosynthesis research. Angew Chem Int Ed Engl 30: 1621–1633
Droillard MJ, Bate NJ, Rothstein S, Thompson, JE (1992) Active translation of the D1 protein of photosystem II in senescing leaves. Plant Physiol 99: 589–594
Eckert H-J, Liu B, Geiken B, Eichler H-J, Renger G (1992) Photoinactivation of electron transfer through PSII as studied by time resolved reaction centre proteins as a function of light. Photosynthetica 27: 355–368
Elich TD, Edelman M, Mattoo AK (1992) Identification, characterization and resolution of the in vivo phosphorylated form of the D1 photosystem II reaction center protein. J Biol Chem 267: 3523–3529
Elich TD, Edelman M, Mattoo AK (1993) Dephosphorylation of photosystem II core proteins is light-regulated in vivo. EMBO J 12: 4857–62
Geiken B, Critchley C, Renger G (1992) The turnover of photosystem II reaction centre proteins as a function of light. In: Murata N (ed) Research in photosynthesis, vol 4. Kluwer Academic Publishers, Dordrecht, pp 643–646
Genty B, Briantais J-M, Vieira da Silva JB (1987) Effects of drought on primary photosynthetic processes of cotton leaves. Plant Physiol 83: 360–364
Giardi MT (1993a) Phosphorylation and disassembly of photosystem II core as an early stage of photoinhibition. Planta 190: 107–113
Giardi MT (1993b) Significance of photosystem II core phosphorylation heterogeneity for the herbicide binding domain. Z Naturforsch 48c: 241–245
Giardi MT, Rigoni F, Barbato R, Giacometti GM (1991) Relationships between heterogeneity of PSII in grana particles in vitro and phosphorylation. Biochem Biophys Res Comm 176: 1298–1305
Giardi MT, Rigoni F, Barbato R (1992) PSII core phosphorylation heterogeneity: differential herbicide binding and electron transfer regulation in PSII particles from spinach. Plant Physiol 100: 1948–1954
Giardi MT, Komenda J, Masojídek J (1994) Involvement of PSII phosphorylation in the sensitivity to strong light. Physiol Plant 92: 181–187
Godde D, Hefer M (1994) Photoinhibition and light dependent turnover of D1 reaction-centre polypeptide of photosystem II are enhanced by mineral-stress conditions. Planta 193, 290–299
Godde D, Schmitz H, Weiner M (1991) Turnover of the D1 reaction center polypeptide from photosystem II in intact spruce needles and spinach leaves. Z Naturforsch 46c: 245–251
Havaux M (1992) Stress tolerance of photosystem II in vivo. Plant Physiol 100: 424–432
Horton P, Lee P (1985) Phosphorylation of chloroplast membrane proteins partially protects against photoinhibition. Planta 165: 37–42
Horton P, Ruban AV (1992) Regulation of PSII. Photosynth Res 34: 375–385
Hurry VM, Gardeström P, Öquist G (1993) Reduced sensitivity to photoinhibition following frost-hardening of winter rye is due to increased phosphate availability. Planta 190: 484–490
Jefferies RA (1994) Drought and chlorophyll fluorescence in fieldgrown potato (Solanum tuberosus). Physiol Plant 90: 93–97
Kaiser WM (1987) Effects of water deficit on photosynthetic capacity. Physiol Plant 71: 142–149
Lichtenthaler HK, Wellburn AR (1983) Determination of total carotenoids and chlorophyll a and b of leaf extracts in different solvents. Biochem Soc Trans 603: 591–592
Masojídek J, Hall DO (1992) Salinity and drought stresses are amplified by high irradiance in sorghum. Photosynthetica 27: 159–171
Masojídek J, Trivedi S, Haishaw L, Alexiou A, Hall DO (1991) The synergistic effect of drought and light stresses in sorghum and pearl millet. Plant Physiol 96: 198–207
Mattoo AK, Edelman M (1985) Photoregulation and metabolism of a thylakoidal herbicide-receptor protein. In: John SB (ed) Frontiers of membrane research in agriculture. Rowman and Allanheld, Totowa, pp 23–24
Mattoo AK, Marder JB, Edelman M (1989) Dynamics of the photosystem II reaction center. Cell 56: 241–246
Meyer S, de Kouchkovsky Y (1993) Electron transport, photosystem-2 reaction centers and chlorophyll-protein complexes of thylakoids of drought resistant and sensitive lupin plants. Photosynth Res 37: 49–60
Ögren E, Öquist G (1984) Photoinhibition of photosynthesis in Lemna gibba as induced by the interaction between light and temperature. III Chlorophyll fluorescence at 77 K. Physiol Plant 62: 193–200
Öquist G, Chow WS, Anderson M (1992) Photoinhibition of photosynthesis represents a mechanism for the long-term regulation of photosystem II. Planta 186: 450–460
Osmond B (1994) What is photoinhibition: some insights from comparisons of sun and shade plants. In: Baker NR, Bowyer JR (eds) Photoinhibition: Molecular mechanisms to the field. Bios Science Publisher, Oxford, pp 1–24
Powles SB (1984) Photoinhibition of photosynthesis induced by visible light. Annu Rev Plant Physiol 35: 15–44
Russell AW, Critchley C, Robinson SA, Franklin LA, Seaton, GGR, Chow WS, Anderson JM, Osmond CB (1995) Photosystem II regulation and dynamics of the chloroplast D1 protein in Arabidopsis leaves during photosynthesis and photoinhibition. Plant Physiol 107: 943–952
Sundby C, Mc Caffery S, Anderson JM (1993) Turnover of the photosystem II D1 protein in higher plants under photoinhibitory and non-photoinhibitory irradiance. J Biol Chem 268: 25476–25482
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by the Italian National Council of Research special grant RAISA, subproject 2 (paper No. 2179) on water stress B. Geiken was supported by the European program “Human Capital and Mobility”. We thank Dr. Roberto Barbato (Department of Biology, University of Padua, Italy) for generous gifts of various PSII antibodies.
Rights and permissions
About this article
Cite this article
Giardi, M.T., Cona, A., Geiken, B. et al. Long-term drought stress induces structural and functional reorganization of photosystem II. Planta 199, 118–125 (1996). https://doi.org/10.1007/BF00196888
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00196888