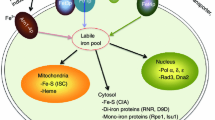
Abstract
Following hydrogen peroxide treatment, ferrous iron (Fe2+) is oxidized to its ferric form (Fe3+), stripping it from and inactivating iron-containing proteins. Many mononuclear iron enzymes can be remetallated by manganese to restore function, while other enzymes specifically utilize manganese as a cofactor, having redundant activities that compensate for iron-depleted counterparts. DNA replication relies on one or more iron-dependent protein(s) as synthesis abates in the presence of hydrogen peroxide and requires manganese in the medium to resume. Here, we show that manganese transporters regulate the ability to resume replication following oxidative challenge in Escherichia coli. The absence of the primary manganese importer, MntH, impairs the ability to resume replication; whereas deleting the manganese exporter, MntP, or transporter regulator, MntR, dramatically increases the rate of recovery. Unregulated manganese import promoted recovery even in the absence of Fur, which maintains iron homeostasis. Similarly, replication was not restored in oxyR mutants, which cannot upregulate manganese import following hydrogen peroxide stress. Taken together, the results define a central role for manganese transport in restoring replication following oxidative stress.






Similar content being viewed by others
References
Andreini C, Bertini I, Cavallaro G, Holliday GL, Thornton JM (2008) Metal ions in biological catalysis: from enzyme databases to general principles. J Biol Inorg Chem 13:1205–1218
Angerer A, Braun V (1998) Iron regulates transcription of the Escherichia coli ferric citrate transport genes directly and through the transcription initiation proteins. Arch Microbiol 169:483–490
Anjem A, Imlay JA (2012) Mononuclear iron enzymes are primary targets of hydrogen peroxide stress. J Biol Chem 287:15544–15556
Anjem A, Varghese S, Imlay JA (2009) Manganese import is a key element of the OxyR response to hydrogen peroxide in Escherichia coli. Mol Microbiol 72:844–858
Aslund F, Zheng M, Beckwith J, Storz G (1999) Regulation of the OxyR transcription factor by hydrogen peroxide and the cellular thiol-disulfide status. Proc Natl Acad Sci U S A 96:6161–6165
Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko KA, Tomita M, Wanner BL, Mori H (2006) Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol 2:20060008
Bagg A, Neilands JB (1987) Ferric uptake regulation protein acts as a repressor, employing iron (II) as a cofactor to bind the operator of an iron transport operon in Escherichia coli. Biochemistry 26:5471–5477
Beyer WF, Fridovich I (1991) In vivo competition between iron and manganese for occupancy of the active site region of the manganese-superoxide dismutase of Escherichia coli. J Biol Chem 266:303–308
Bosma EF, Rau MH, van Gijtenbeek LA, Siedler S (2021) Regulation and distinct physiological roles of manganese in bacteria. FEMS Microbiol Rev 45:8468
Castellani A, Jagger J, Setlow RB (1964) Overlap of photoreactivation and liquid holding recovery in Escherichia coli B. Science 143:1170–1171
Chow KH, Courcelle J (2004) RecO acts with RecF and RecR to protect and maintain replication forks blocked by UV-induced DNA damage in Escherichia coli. J Biol Chem 279:3492–3496
Cotruvo JA, Stubbe J (2011) Class I ribonucleotide reductases: metallocofactor assembly and repair in vitro and in vivo. Annu Rev Biochem 80:733–767
Courcelle J, Hanawalt PC (2001) Participation of recombination proteins in rescue of arrested replication forks in UV-irradiated Escherichia coli need not involve recombination. Proc Natl Acad Sci U S A 98:8196–8202
Courcelle J, Carswell-Crumpton C, Hanawalt PC (1997) recF and recR are required for the resumption of replication at DNA replication forks in Escherichia coli. Proc Natl Acad Sci U S A 94:3714–3719
Courcelle J, Crowley DJ, Hanawalt PC (1999) Recovery of DNA replication in UV-irradiated Escherichia coli requires both excision repair and recF protein function. J Bacteriol 181:916–922
Courcelle J, Ganesan AK, Hanawalt PC (2001) Therefore, what are recombination proteins there for? BioEssays 23:463–470
Courcelle J, Donaldson JR, Chow KH, Courcelle CT (2003) DNA damage-induced replication fork regression and processing in Escherichia coli. Science 299:1064–1067
Cvetkovic A, Menon AL, Thorgersen MP, Scott JW, Poole FL, Jenney FE, Lancaster WA, Praissman JL, Shanmukh S, Vaccaro BJ, Trauger SA, Kalisiak E, Apon JV, Siuzdak G, Yannone SM, Tainer JA, Adams MW (2010) Microbial metalloproteomes are largely uncharacterized. Nature 466:779–782
Daly MJ, Gaidamakova EK, Matrosova VY, Vasilenko A, Zhai M, Venkateswaran A, Hess M, Omelchenko MV, Kostandarithes HM, Makarova KS, Wackett LP, Fredrickson JK, Ghosal D (2004) Accumulation of Mn(II) in Deinococcus radiodurans facilitates gamma-radiation resistance. Science 306:1025–1028
Daly MJ, Gaidamakova EK, Matrosova VY, Vasilenko A, Zhai M, Leapman RD, Lai B, Ravel B, Li SM, Kemner KM, Fredrickson JK (2007) Protein oxidation implicated as the primary determinant of bacterial radioresistance. PLoS Biol 5:e92
Dambach M, Sandoval M, Updegrove TB, Anantharaman V, Aravind L, Waters LS, Storz G (2015) The ubiquitous yybp-ykoy riboswitch is a manganese-responsive regulatory element. Mol Cell 57:1099–1109
Datsenko KA, Wanner BL (2000) One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 97:6640–6645
Faulkner MJ, Helmann JD (2010) Peroxide stress elicits adaptive changes in bacterial metal ion homeostasis. Antioxid Redox Signal. https://doi.org/10.1089/ars.2010.3682
Fontenot CR, Tasnim H, Valdes KA, Popescu CV, Ding H (2020) Ferric uptake regulator (Fur) reversibly binds a [2Fe–2S] cluster to sense intracellular iron homeostasis in. J Biol Chem 295:15454–15463
Ganesan AK, Smith KC (1969) Dark recovery processes in Escherichia coli irradiated with ultraviolet light. II. Effect of uvr genes on liquid holding recovery. J Bacteriol 97:1129–1133
Haber F, Weiss J (1934) The Catalytic decomposition of hydrogen peroxide by iron salts. Proc R Soc London Ser A 147:332–351
Hassan HM, Fridovich I (1977) Enzymatic defenses against the toxicity of oxygen and of streptonigrin in Escherichia coli. J Bacteriol 129:1574–1583
Hoff CA, Schmidt SS, Hackert BJ, Worley TK, Courcelle J, Courcelle CT (2021) Events associated with DNA replication disruption are not observed in hydrogen peroxide-treated Escherichia coli. G3 (Bethesda). https://doi.org/10.1093/g3journal/jkab044
Howard-Flanders P, Theriot L, Stedeford JB (1969) Some properties of excision-defective recombination-deficient mutants of Escherichia coli K-12. J Bacteriol 97:1134–1141
Hutfilz CR, Wang NE, Hoff CA, Lee JA, Hackert BJ, Courcelle J, Courcelle CT (2019) Manganese is required for the rapid recovery of DNA synthesis following oxidative challenge in. J Bacteriol. https://doi.org/10.1128/jb.00426-19
Imlay JA (2014) The mismetallation of enzymes during oxidative stress. J Biol Chem 289:28121–28128
Imlay JA, Linn S (1986) Bimodal pattern of killing of DNA-repair-defective or anoxically grown Escherichia coli by hydrogen peroxide. J Bacteriol 166:519–527
Imlay JA, Fridovich I (1991) Assay of metabolic superoxide production in Escherichia coli. J Biol Chem 266:6957–6965
Imlay JA, Chin SM, Linn S (1988) Toxic DNA damage by hydrogen peroxide through the Fenton reaction in vivo and in vitro. Science 240:640–642
Kaur G, Sengupta S, Kumar V, Kumari A, Ghosh A, Parrack P, Dutta D (2014) Novel MntR-Independent mechanism of manganese homeostasis in Escherichia coli by the ribosome-associated protein HflX. J Bacteriol 196:2587–2597
Kehres DG, Maguire ME (2003) Emerging themes in manganese transport, biochemistry and pathogenesis in bacteria. FEMS Microbiol Rev 27:263–290
Kehres DG, Zaharik ML, Finlay BB, Maguire ME (2000) The NRAMP proteins of Salmonella typhimurium and Escherichia coli are selective manganese transporters involved in the response to reactive oxygen. Mol Microbiol 36:1085–1100
Kehres DG, Janakiraman A, Slauch JM, Maguire ME (2002) Regulation of Salmonella enterica serovar Typhimurium mntH transcription by H(2)O(2), Fe(2+), and Mn(2+). J Bacteriol 184:3151–3158
Keyer K, Imlay JA (1996) Superoxide accelerates DNA damage by elevating free-iron levels. Proc Natl Acad Sci U S A 93:13635–13640
Levin JD, Shapiro R, Demple B (1991) Metalloenzymes in DNA repair. Escherichia coli endonuclease IV and Saccharomyces cerevisiae Apn1. J Biol Chem 266:22893–22898
Li Z, Demple B (1994) SoxS, an activator of superoxide stress genes in Escherichia coli. Purification and interaction with DNA. J Biol Chem 269:18371–18377
Lisova AE, Baranovskiy AG, Morstadt LM, Babayeva ND, Stepchenkova EI, Tahirov TH (2022) The iron-sulfur cluster is essential for DNA binding by human DNA polymerase ε. Sci Rep 12:17436
Makui H, Roig E, Cole ST, Helmann JD, Gros P, Cellier MF (2000) Identification of the Escherichia coli K-12 nramp orthologue (MntH) as a selective divalent metal ion transporter. Mol Microbiol 35:1065–1078
Martin JE, Imlay JA (2011) The alternative aerobic ribonucleotide reductase of Escherichia coli, NrdEF, is a manganese-dependent enzyme that enables cell replication during periods of iron starvation. Mol Microbiol 80:319–334
Martin JE, Waters LS (2022) Regulation of bacterial manganese homeostasis and usage during stress responses and pathogenesis. Front Mol Biosci 9:945724
Martin JE, Waters LS, Storz G, Imlay JA (2015) The Escherichia coli small protein MntS and exporter MntP optimize the intracellular concentration of manganese. PLoS Genet 11:e1004977
McHugh JP, Rodríguez-Quinoñes F, Abdul-Tehrani H, Svistunenko DA, Poole RK, Cooper CE, Andrews SC (2003) Global iron-dependent gene regulation in Escherichia coli. A new mechanism for iron homeostasis. J Biol Chem 278:29478–29486
Netz DJ, Stith CM, Stümpfig M, Köpf G, Vogel D, Genau HM, Stodola JL, Lill R, Burgers PM, Pierik AJ (2011) Eukaryotic DNA polymerases require an iron-sulfur cluster for the formation of active complexes. Nat Chem Biol 8:125–132
Ona KR, Courcelle CT, Courcelle J (2009) Nucleotide excision repair is a predominant mechanism for processing nitrofurazone-induced DNA damage in Escherichia coli. J Bacteriol 191:4959–4965
Patzer SI, Hantke K (2001) Dual repression by Fe(2+)-Fur and Mn(2+)-MntR of the mntH gene, encoding an NRAMP-like mn(2+) transporter in Escherichia coli. J Bacteriol 183:4806–4813
Rupp WD, Howard-Flanders P (1968) Discontinuities in the DNA synthesized in an excision-defective strain of Escherichia coli following ultraviolet irradiation. J Mol Biol 31:291–304
Seib KL, Tseng HJ, McEwan AG, Apicella MA, Jennings MP (2004) Defenses against oxidative stress in Neisseria gonorrhoeae and Neisseria meningitidis: distinctive systems for different lifestyles. J Infect Dis 190:136–147
Seo SW, Kim D, Latif H, O’Brien EJ, Szubin R, Palsson BO (2014) Deciphering Fur transcriptional regulatory network highlights its complex role beyond iron metabolism in Escherichia coli. Nat Commun 5:4910
Seo SW, Kim D, Szubin R, Palsson BO (2015) Genome-wide reconstruction of OxyR and SoxRS transcriptional regulatory networks under oxidative stress in Escherichia coli K-12 MG1655. Cell Rep 12:1289–1299
Sobota JM, Imlay JA (2011) Iron enzyme ribulose-5-phosphate 3-epimerase in Escherichia coli is rapidly damaged by hydrogen peroxide but can be protected by manganese. Proc Natl Acad Sci U S A 108:5402–5407
Storz G, Tartaglia LA, Ames BN (1990) Transcriptional regulator of oxidative stress-inducible genes: direct activation by oxidation. Science 248:189–194
Tamarit J, Cabiscol E, Ros J (1998) Identification of the major oxidatively damaged proteins in Escherichia coli cells exposed to oxidative stress. J Biol Chem 273:3027–3032
Touati D, Jacques M, Tardat B, Bouchard L, Despied S (1995) Lethal oxidative damage and mutagenesis are generated by iron in delta fur mutants of Escherichia coli: protective role of superoxide dismutase. J Bacteriol 177:2305–2314
Tseng HJ, Srikhanta Y, McEwan AG, Jennings MP (2001) Accumulation of manganese in Neisseria gonorrhoeae correlates with resistance to oxidative killing by superoxide anion and is independent of superoxide dismutase activity. Mol Microbiol 40:1175–1186
Tseng HJ, McEwan AG, Paton JC, Jennings MP (2002) Virulence of Streptococcus pneumoniae: PsaA mutants are hypersensitive to oxidative stress. Infect Immun 70:1635–1639
Varghese S, Wu A, Park S, Imlay KR, Imlay JA (2007) Submicromolar hydrogen peroxide disrupts the ability of Fur protein to control free-iron levels in Escherichia coli. Mol Microbiol 64:822–830
Waters LS (2020) Bacterial manganese sensing and homeostasis. Curr Opin Chem Biol 55:96–102
Waters LS, Sandoval M, Storz G (2011) The Escherichia coli MntR miniregulon includes genes encoding a small protein and an efflux pump required for manganese homeostasis. J Bacteriol 193:5887–5897
Wu J, Weiss B (1992) Two-stage induction of the soxRS (superoxide response) regulon of Escherichia coli. J Bacteriol 174:3915–3920
Yamamoto K, Ishihama A, Busby SJ, Grainger DC (2011) The Escherichia coli K-12 MntR miniregulon includes dps, which encodes the major stationary-phase DNA-binding protein. J Bacteriol 193:1477–1480
Yousuf S, Karlinsey JE, Neville SL, McDevitt CA, Libby SJ, Fang FC, Frawley ER (2020) Manganese import protects Salmonella enterica serovar Typhimurium against nitrosative stress. Metallomics 12:1791–1801
Zheng M, Storz G (2000) Redox sensing by prokaryotic transcription factors. Biochem Pharmacol 59:1–6
Zheng M, Doan B, Schneider TD, Storz G (1999) OxyR and SoxRS regulation of fur. J Bacteriol 181:4639–4643
Zheng M, Wang X, Templeton LJ, Smulski DR, LaRossa RA, Storz G (2001) DNA microarray-mediated transcriptional profiling of the Escherichia coli response to hydrogen peroxide. J Bacteriol 183:4562–4570
Acknowledgements
The authors thank Ben Perkins in the Trace Element Analytical Laboratory at Portland State University for assistance with ICP-MS analysis.
Funding
This study was supported by Grant No. MCB1916625 from the National Science Foundation, R21ES034880 from the NIH National Institute of Environmental Health Science, and R16GM14554 from the NIH National Institute of General Medical Sciences.
Author information
Authors and Affiliations
Contributions
NEW, CTC and JC conceptualization; NEW, CTC and JC methodology; NEW, EJC, SMC, RLS, CTC and JC investigation; NEW, EJC, SMC, RLS, CTC and JC data analysis; NEW, CTC and JC writing original draft; NEW, EJC, CTC and JC writing review and editing; JC funding acquisition.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant conflicts of interest to disclose.
Additional information
Publisher’s Note
Springer nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, N.E., Courcelle, E.J., Coltman, S.M. et al. Manganese transporters regulate the resumption of replication in hydrogen peroxide-stressed Escherichia coli. Biometals 36, 1361–1376 (2023). https://doi.org/10.1007/s10534-023-00523-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-023-00523-8