Abstract
Serum zinc has been implicated as an important mediator of haemostasis and thrombosis. However, the nature and magnitude of any potential relationship between serum zinc and venous thromboembolism (VTE) is unknown. We aimed to evaluate the prospective association between serum zinc and VTE risk. We analyzed data involving 2472 men aged 42–61 years without a history of VTE in the Kuopio Ischemic Heart Disease population-based cohort study, with the assessment of serum zinc concentrations using atomic absorption spectrometry. Hazard ratios (95% confidence intervals [CIs]) for incident VTE were estimated. A total of 166 VTE cases occurred during a median follow-up of 27.1 years. The risk of VTE per 1 standard deviation increase in serum zinc in analysis adjusted for systolic blood pressure, body mass index, total cholesterol, triglycerides, smoking status, histories of type 2 diabetes and coronary heart disease, medication for dyslipidaemia, alcohol consumption, physical activity, and socioeconomic status was (HR 1.03; 95% CI 0.86–1.22), which remained similar (HR 1.04; 95% CI 0.87–1.23) following further adjustment for inflammation and history of cancer. Comparing the extreme tertiles of serum zinc, the corresponding adjusted HRs (95% CIs) were 0.92 (0.63–1.36) and 0.94 (0.64–1.39), respectively. Imputed results based on 2682 participants and 176 VTE events were consistent with the observed results. In middle-aged and older Finnish men, serum zinc is not associated with future VTE risk. Other large-scale prospective studies conducted in other populations are needed to confirm or refute these findings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardiovascular disease (CVD) persists as the leading cause of death globally despite major advances in the development and implementation of preventive and management strategies (Barquera et al. 2015). Cardiovascular diseases are also associated with substantial morbidity and costs to healthcare systems and economies. Atherosclerotic CVDs (arterial thrombotic disease), which include coronary heart disease (CHD) and cerebrovascular disease (ischemic stroke) (Barquera et al. 2015) are the major manifestations of CVD. Atherosclerotic CVD is closely related to venous thromboembolism (VTE), a vascular condition which comprises deep vein thrombosis (DVT) and pulmonary embolism (PE)). Both disease entities share mechanistic pathways such as coagulation, platelet activation and dyslipidaemia (Ray 2003) and common risk factors including age, obesity and cigarette smoking (Ageno et al. 2008; Glynn and Rosner 2005). Venous thromboembolism is also associated with significant morbidity, high economic costs and is a preventable cause of death (Cohen et al. 2007; Douketis et al. 2007).
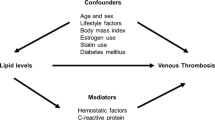
Zinc, the second most abundant trace metal in the body, is an essential micronutrient that is involved in several cellular processes. It is involved in nucleic acid synthesis, enzymatic reactions, cell replication and repair, and also plays an important role in energy producing functions (Chimienti 2013). Zinc has antioxidant and anti-inflammatory properties (Jarosz et al. 2017; Olechnowicz et al. 2018). Zinc deficiency leads to many disorders. Evidence indicates that circulating levels of zinc may be involved in regulation of blood pressure (Tubek 2007) and also exhibit cardio-protective effects. Observational cohort studies have demonstrated associations between serum zinc and risk of hypertension and CVD (Kok et al. 1988; Kunutsor and Laukkanen 2016; Reunanen et al. 1996). There is mounting evidence that zinc is an important mediator of haemostasis and thrombosis. Zinc binds numerous plasma proteins and modulates their structure and function, and its deficiency is associated with bleeding and clotting abnormalities (Vu et al. 2013). Zinc is an endogenous and exogenous regulator of platelet function during haemostasis and thrombosis; extracellular zinc ions (Zn2+) gain access to the platelet cytosol and induce full platelet activation at high concentrations (Ahmed et al. 2021). Activated platelets also secrete zinc into the local microenvironment, with increased concentrations of zinc observed in the vicinity of a thrombus (Vu et al. 2013).
Given the linked pathways between zinc, atherosclerotic CVD, and VTE, and the biological plausibility that serum zinc may be involved in the development of VTE, we hypothesized that a potential association may exist between serum zinc and the risk of VTE. The nature and magnitude of any association between serum zinc and VTE risk using a prospective study has not been previously explored. In this context, we aimed to evaluate the prospective association between serum zinc and VTE risk using a population-based prospective cohort of 2,472 middle-aged and older Finnish men.
Methods
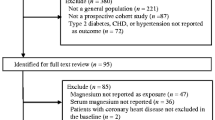
Reporting of the study conforms to broad EQUATOR guidelines (Simera et al. 2010) and was conducted according to STROBE (STrengthening the Reporting of OBservational studies in Epidemiology) guidelines for reporting observational studies in epidemiology (Supplementary File 1). The Research Ethics Committee of the University of Eastern Finland approved the study (reference #:143/97), and each participant gave written informed consent. All study procedures were conducted according to the Declaration of Helsinki. Study participants were part of the Kuopio Ischemic Heart Disease (KIHD) risk factor study, a longitudinal population-based study designed to investigate risk factors for atherosclerotic CVD and other chronic diseases. Participants were a representative sample of randomly selected men living in the city of Kuopio and its surrounding rural communities in Eastern Finland. Baseline examinations were performed between 1984 and 1989 and included men 42–61 years of age. Study design and recruitment methods have been described in detail in previous reports (Kunutsor et al. 2019a, 2019b, 2020, 2018). The current analysis is based on 2472 men with no previous history of VTE and non-missing data on serum zinc, relevant covariates, and first VTE events.
The assessment of risk markers and other covariates have been described previously (Kunutsor et al. 2019a, 2019b, 2020, 2018). Besides fasting overnight, participants were told to abstain from drinking alcohol for at least 3 days and from smoking for at least 12 h before blood samples were taken between 8 and 10 a.m. Measurements of serum zinc concentrations were made from frozen serum samples stored at – 20 °C for 1–5 years. The PerkinElmer 306 atomic absorption spectrophotometer (Norwalk, Connecticut, USA) was used for the measurements, which employed a flame technique and pyrolytically coated graphite tubes with a platform (Salonen et al. 1991). Seronorm (Nycomed, Oslo, Norway) control serum samples were included in all daily batches. The reference standards were dissolved in 5% glycerol and the between-batch coefficient of variation was 4.0%. All incident VTE events that occurred from study entry to 2018 were included. All VTE events required positive imaging tests for their diagnoses and were identified by computer linkage to the National Hospital Discharge Registry data maintained by the Finnish Institute for Health and Welfare and their diagnoses. Each VTE event was validated by two physicians following detailed cross-checking of medical documents. The ICD 10 codes (I26, I80 and I82) were used to code and classify each VTE case. Hazard ratios (HRs) with 95% confidence intervals (CIs) for incident VTE were estimated using Cox proportional hazard models. The adjustment for confounders were based on four models: (Model 1) age; (Model 2) Model 1 plus systolic blood pressure (SBP), body mass index (BMI), total cholesterol, triglycerides, smoking status, history of type 2 diabetes (T2D), history of coronary heart disease (CHD), medication for dyslipidaemia, alcohol consumption, physical activity, and socioeconomic status (SES); (Model 3) Model 2 plus high sensitivity C-reactive protein (hsCRP) and history of cancer; and (Model 4) a model comprising dietary factors including serum magnesium, total energy intake, intake of processed and unprocessed red meat, and intake of fruits, berries and vegetables. The confounders selected were based on their previously established roles as risk factors for VTE, evidence from previous research, previously published associations with VTE in the KIHD study (Kunutsor et al. 2021, 2019a; Kunutsor and Laukkanen 2021), or their potential as confounders based on known associations with VTE outcomes and observed associations with serum zinc using the available data (Groenwold et al. 2011). Given the long-follow-up of the cohort, we explored the potential for regression dilution bias by conducting sensitivity analysis that was restricted to the first 10 years of follow-up. Finally, multiple imputation by chained equations (MICE) was conducted to handle potential selection bias originating from missingness. The imputation model included all model covariates as well as VTE outcome status. Ten imputations were computed due to the computational time required. Cox regression analyses were run across the 10 imputed datasets and the pooled estimates were reported. All statistical analyses were conducted using Stata version MP 16 (Stata Corp, College Station, Texas).
Results
Table 1 shows baseline characteristics of study participants overall and by VTE development at end of follow-up. The mean [standard deviation (SD)] age and serum zinc of the 2472 men at baseline were 53 (5) years and 0.94 (0.12) mg/l, respectively. Levels of most of the risk markers including serum zinc were similar between those who developed and did not develop VTE.
A total of 166 VTE cases occurred during a median (interquartile range) follow-up of 27.1 (17.2–31.0) years, which corresponded to an annual rate of 2.85/1000 person-years at risk (95% CI 2.45 to 3.32). In age-adjusted analysis, the HR (95% CI) for VTE per 1 SD increase in serum zinc was 1.05 (0.89–1.24), which remained similar 1.03 (0.86–1.22) on further adjustment for SBP, BMI, total cholesterol, triglycerides, smoking status, histories of T2D and coronary heart disease (CHD), medication for dyslipidaemia, alcohol consumption, physical activity, and SES. Additional adjustment for hsCRP and history of cancer did not attenuate the association 1.04 (0.87–1.23) (Table 2). Comparing the extreme tertiles of serum zinc, the corresponding adjusted HRs (95% CIs) were 0.99 (0.68–1.45), 0.92 (0.63–1.36) and 0.94 (0.64–1.39), respectively. In the model that comprised dietary factors, the association persisted (Table 2). In sensitivity analysis restricted to the first 10 years of follow-up, the results were consistent (Supplementary File 2). Data was imputed for 2682 participants with 176 VTE events and the imputed results were consistent with those obtained using observed values (Supplementary File 3).
Discussion
Given the potential interplay between zinc, atherosclerotic CVD, and VTE, and that zinc may be involved in haemostasis and thrombosis, we sought to investigate the potential prospective relationship between serum zinc and VTE risk in a general population-based cohort of middle-aged and older Finnish men. Our findings showed that increased serum levels of zinc were not associated with the future risk of VTE. Results were similar in analyses restricted to the first 10 years of follow-up. Furthermore, imputed results were similar to the observed results. A detailed literature search did not identify any previous studies that have evaluated the association between zinc status and VTE. Hence, it is difficult to discuss these findings in the context of previous studies. Other large-scale studies are warranted to refute or confirm these findings.
Zinc is an essential trace element with antioxidant and anti-inflammatory activities (Jansen et al. 2009). Growing evidence derived from cell and animal studies supports a cardioprotective role of zinc. Zinc deficiency has been shown to elicit the release of pro-atherogenic factors (Reiterer et al. 2005) and supplementation with zinc has been shown to reduce atheroma formation and plasma and arterial wall lipid peroxidation and also decrease the incidence of arrhythmias (Little et al. 2010). Zinc regulates vascular endothelial cell activity (Zhu et al. 2018) and has also been implicated in arterial thrombosis (Mammadova-Bach and Braun 2019). Consistent with the mechanistic evidence, a number of observational studies have shown higher serum zinc levels or dietary zinc intakes to be associated with reduced risk of CVD (Bates et al. 2011; Kok et al. 1988; Pilz et al. 2009; Reunanen et al. 1996; Soinio et al. 2007) and better recovery of neurological deficits following an ischemic stroke (Aquilani et al. 2009). In addition to its several physiological functions, zinc has been reported to be an important mediator of haemostasis and thrombosis by modulating coagulation, platelet aggregation, anticoagulation and fibrinolysis (Vu et al. 2013). Platelet function and activation is dependent on zinc; zinc deficiency results in prolonged bleeding and reduced platelet aggregation (Mammadova-Bach and Braun 2019). Similar to calcium, zinc enhances thrombin-induced fibrin clot formation, an essential step in the haemostatic process (Mammadova-Bach and Braun 2019). It has been reported that platelet accumulation and activation at the sites of vascular injury lead to the release of zinc ions from platelets to the microenvironment of the vascular network; (Mammadova-Bach and Braun 2019) experimental evidence suggests that platelet-resident zinc may modulate the process of thrombosis, but there is uncertainty how this is achieved (Mammadova-Bach and Braun 2019).
The null findings may seem unexpected for the following reasons: (i) the close relationship between atherosclerotic CVD and VTE via shared risk factors and pathophysiological pathways and previous evidence of associations between zinc status and atherosclerotic CVD and (ii) the wealth of evidence showing that zinc may be an important co-factor in thrombosis. On the contrary, the null findings may reflect the true relationship between zinc status and future VTE risk. Zinc may represent one of the many factors involved in the thrombotic process and may not necessarily be a risk marker for VTE. These findings may also suggest important differences in the aetiology and pathophysiology of arterial thrombotic disease and VTE. Though these two disease states may be closely linked, they may in fact be two distinct diseases as viewed historically (Prandoni 2007). The evidence has not been very consistent. Whiles some studies have reported that atherosclerotic CVD is an underlying condition and precedes the development of VTE (Prandoni et al. 2003), other studies have shown that atherosclerotic CVD does not precede VTE development (Reich et al. 2006; van der Hagen et al. 2006) or VTE rather precedes atherosclerotic CVD (Prandoni et al. 2006). With regards to shared risk factors, some studies have demonstrated associations between traditional CVD risk factors and VTE risk (Ageno et al. 2008; Gregson et al. 2019), whereas, others have not (Mahmoodi et al. 2017; Wattanakit et al. 2012) Other factors that could potentially explain our null findings could be due to the population characteristics such as male only sex and the age group (middle-aged and older). The low event rate may have provided low power to detect an association. Finally, there was a potential for regression dilution bias due to availability of only single baseline measurements of zinc and the particularly long follow-up duration of the cohort, which could have underestimated the true strength of the association. (Horvei et al. 2016; Smabrekke et al. 2016) Consistent with the phenomenon of regression dilution bias, several cohort studies evaluating associations between exposures and outcomes have demonstrated significant evidence of associations at short-term follow-up, with no evidence of associations at long-term follow-up (Kunutsor et al. 2017; Quist-Paulsen et al. 2010). We attempted to explore for evidence of this bias in our cohort by restricting analysis to the first 10 years of follow-up, but the null association between serum zinc and VTE risk persisted albeit in the presence of a low event rate.
To our knowledge, this is the first study to evaluate if a temporal relationship exists between zinc status and VTE risk. Other strengths include the population-based prospective cohort design, inclusion of a random representative sample of men from an ethnically and genetically homogeneous population, the follow-up duration which was long enough for the ascertainment of VTE events, ability to adjust for a comprehensive list of risk markers for VTE, and multiple imputation to account for missing data. The limitations included inability to generalise the findings to men and other populations, lack of data on VTE subtypes and potential confounders such as history of inflammatory bowel disease and other gastrointestinal disorders, and the potential for residual confounding due to the observational design. The prolonged storage of serum samples (1–5 years) could have affected the stability of the samples. However, zinc concentrations have been shown not to be affected by prolonged storage in frozen serum samples (at – 20 ℃) for several years or repeated freeze–thaw cycles (Arnaud 2010; Pirkle 2013). We used single baseline measurements of zinc and could therefore not correct for regression dilution bias, which potentially results in the underestimation of the true association between an exposure and outcome, particularly for cohort studies with long-term follow-up (Horvei et al. 2016; Smabrekke et al. 2016). Furthermore, serum or plasma zinc concentrations may not accurately reflect actual zinc status. There is substantial interindividual variability in blood zinc concentrations with changes in dietary zinc; they respond quickly to zinc supplementation between meals than additional zinc provided in food, and are also influenced by time of the day, recent meal consumption, inflammation, hormones and certain drugs (King et al. 2015). Furthermore, given that albumin is the primary carrier protein for circulating zinc, measured zinc concentrations may not reflect actual status in some populations such as those with acute illness or malnutrition (King et al. 2015). However, given the limited data available on hair, urinary, nail, and blood cell zinc responses to changes in dietary zinc, the Biomarkers of Nutrition for Development (BOND) Zinc Expert Panel recommends the use of plasma zinc concentration as a biomarker of zinc status (King et al. 2015). Despite the many limitations and constraints of plasma zinc, it has been reported as the only biomarker of status that can be used to measure zinc status in individuals with either a low or a high supply of dietary zinc (King et al. 2015). Given that this is the first evaluation of the topic and in addition to the limitations, the findings should be interpreted with caution and should be regarded as hypothesis generating. We call on investigators with relevant data on the subject data to explore this further.
Conclusions
Serum zinc is not associated with future VTE risk in middle-aged and older Finnish men. Other large-scale prospective studies conducted in other populations are needed to confirm or refute these findings.
Data availability
The data that support the findings of this study are available from the Principal Investigator (J.A.L.) upon reasonable request.
Abbreviations
- BMI:
-
Body mass index
- CHD:
-
Coronary heart disease
- CI:
-
Confidence interval
- CVD:
-
Cardiovascular disease
- DVT:
-
Deep vein thrombosis
- HDL-C:
-
High-density lipoprotein cholesterol
- HR:
-
Hazard ratio
- hsCRP:
-
High-sensitivity C-reactive protein
- KIHD:
-
Kuopio ischemic heart disease
- PE:
-
Pulmonary embolism
- SD:
-
Standard deviation
- VTE:
-
Venous thromboembolism
References
Ageno W, Becattini C, Brighton T, Selby R, Kamphuisen PW (2008) Cardiovascular risk factors and venous thromboembolism: a meta-analysis. Circulation 117:93–102
Ahmed NS, Lopes-Pires M, Pugh N (2021) Zinc: an endogenous and exogenous regulator of platelet function during hemostasis and thrombosis. Platelets 32:880–887
Aquilani R, Baiardi P, Scocchi M, Iadarola P, Verri M, Sessarego P, Boschi F, Pasini E, Pastoris O, Viglio S (2009) Normalization of zinc intake enhances neurological retrieval of patients suffering from ischemic strokes. Nutr Neurosci 12:219–225
Arnaud J (2010) Stability of serum copper, slenium and zinc. http://www.trace-elements.eu/secure/DownloadFile.aspx?File=2010%20Stability_FESTEM%20(poster).pdf. Accessed 31 Aug 2021
Barquera S, Pedroza-Tobias A, Medina C, Hernandez-Barrera L, Bibbins-Domingo K, Lozano R, Moran AE (2015) Global overview of the epidemiology of atherosclerotic cardiovascular disease. Arch Med Res 46:328–338
Bates CJ, Hamer M, Mishra GD (2011) Redox-modulatory vitamins and minerals that prospectively predict mortality in older British people: the National Diet and Nutrition Survey of people aged 65 years and over. Br J Nutr 105:123–132
Chimienti F (2013) Zinc, pancreatic islet cell function and diabetes: new insights into an old story. Nutr Res Rev 26:1–11
Cohen AT, Agnelli G, Anderson FA, Arcelus JI, Bergqvist D, Brecht JG, Greer IA, Heit JA, Hutchinson JL, Kakkar AK, Mottier D, Oger E, Samama MM, Spannagl M et al (2007) (2007) Venous thromboembolism (VTE) in Europe the number of VTE events and associated morbidity and mortality. Thromb Haemost 98:756–764
Douketis JD, Gu CS, Schulman S, Ghirarduzzi A, Pengo V, Prandoni P (2007) The risk for fatal pulmonary embolism after discontinuing anticoagulant therapy for venous thromboembolism. Ann Intern Med 147:766–774
Glynn RJ, Rosner B (2005) Comparison of risk factors for the competing risks of coronary heart disease, stroke, and venous thromboembolism. Am J Epidemiol 162:975–982
Gregson J, Kaptoge S, Bolton T, Pennells L, Willeit P, Burgess S, Bell S, Sweeting M, Rimm EB, Kabrhel C, Zoller B, Assmann G, Gudnason V, Folsom AR, Arndt V, Fletcher A, Norman PE, Nordestgaard BG, Kitamura A, Mahmoodi BK, Whincup PH, Knuiman M, Salomaa V, Meisinger C, Koenig W, Kavousi M, Volzke H, Cooper JA, Ninomiya T, Casiglia E, Rodriguez B, Ben-Shlomo Y, Despres JP, Simons L, Barrett-Connor E, Bjorkelund C, Notdurfter M, Kromhout D, Price J, Sutherland SE, Sundstrom J, Kauhanen J, Gallacher J, Beulens JWJ, Dankner R, Cooper C, Giampaoli S, Deen JF, Gomez-de-la-Camara A, Kuller LH, Rosengren A, Svensson PJ, Nagel D, Crespo CJ, Brenner H, Albertorio-Diaz JR, Atkins R, Brunner EJ, Shipley M, Njolstad I, Lawlor DA, van der Schouw YT, Selmer RM, Trevisan M, Verschuren WMM, Greenland P, Wassertheil-Smoller S, Lowe GDO, Wood AM, Butterworth AS, Thompson SG, Danesh J, Di Angelantonio E, Meade T et al (2019) Cardiovascular risk factors associated with venous thromboembolism. JAMA Cardiol 4:163–173
Groenwold RH, Klungel OH, Grobbee DE, Hoes AW (2011) Selection of confounding variables should not be based on observed associations with exposure. Eur J Epidemiol 26:589–593
Horvei LD, Grimnes G, Hindberg K, Mathiesen EB, Njolstad I, Wilsgaard T, Brox J, Braekkan SK, Hansen JB (2016) C-reactive protein, obesity, and the risk of arterial and venous thrombosis. J Thromb Haemost 14:1561–1571
Jansen J, Karges W, Rink L (2009) Zinc and diabetes–clinical links and molecular mechanisms. J Nutr Biochem 20:399–417
Jarosz M, Olbert M, Wyszogrodzka G, Mlyniec K, Librowski T (2017) Antioxidant and anti-inflammatory effects of zinc. Zinc-dependent NF-kappaB signaling. Inflammopharmacology 25:11–24
King JC, Brown KH, Gibson RS, Krebs NF, Lowe NM, Siekmann JH, Raiten DJ (2015) Biomarkers of nutrition for development (BOND)-zinc review. J Nutr 146:858S-885S
Kok FJ, Van Duijn CM, Hofman A, Van der Voet GB, De Wolff FA, Paays CH, Valkenburg HA (1988) Serum copper and zinc and the risk of death from cancer and cardiovascular disease. Am J Epidemiol 128:352–359
Kunutsor SK, Laukkanen JA (2021) Circulating serum magnesium and the risk of venous thromboembolism in men: a long-term prospective cohort study. Pulse (basel) 8:108–113
Kunutsor SK, Sameul S, Blom AW, Khunti K, Laukkanen JA (2017) Serum C-reactive protein increases the risk of venous thromboembolism: a prospective study and meta-analysis of published prospective evidence. Eur J Epidemiol 32:657–667
Kunutsor SK, Makikallio TH, Voutilainen A, Laukkanen JA (2020) Handgrip strength is not associated with risk of venous thromboembolism: a prospective cohort study. Scand Cardiovasc J 54:253–257
Kunutsor SK, Dey RS, Laukkanen JA (2021) Circulating serum copper is associated with atherosclerotic cardiovascular disease, but not venous thromboembolism: a prospective cohort study. Pulse (basel) 9:109–115
Kunutsor SK, Seidu S, Katechia DT, Laukkanen JA (2018) Inverse association between serum albumin and future risk of venous thromboembolism: interrelationship with high sensitivity C-reactive protein. Ann Med 50:240–248
Kunutsor SK, Makikallio TH, Araujo CGS, Jae SY, Kurl S, Laukkanen JA (2019a) Cardiorespiratory fitness is not associated with risk of venous thromboembolism: a cohort study. Scand Cardiovasc J 53:255–258
Kunutsor SK, Makikallio TH, Khan H, Laukkanen T, Kauhanen J, Laukkanen JA (2019b) Sauna bathing reduces the risk of venous thromboembolism: a prospective cohort study. Eur J Epidemiol 34:983–986
Kunutsor SK, Laukkanen JA (2016) Serum zinc concentrations and incident hypertension: new findings from a population-based cohort study. J Hypertens 34:1055–1061
Little PJ, Bhattacharya R, Moreyra AE, Korichneva IL (2010) Zinc and cardiovascular disease. Nutrition 26:1050–1057
Mahmoodi BK, Cushman M, Anne-Naess I, Allison MA, Bos WJ, Braekkan SK, Cannegieter SC, Gansevoort RT, Gona PN, Hammerstrom J, Hansen JB, Heckbert S, Holst AG, Lakoski SG, Lutsey PL, Manson JE, Martin LW, Matsushita K, Meijer K, Overvad K, Prescott E, Puurunen M, Rossouw JE, Sang Y, Severinsen MT, Ten-Berg J, Folsom AR, Zakai NA (2017) Association of traditional cardiovascular risk factors with venous thromboembolism: an individual participant data meta-analysis of prospective studies. Circulation 135:7–16
Mammadova-Bach E, Braun A (2019) Zinc homeostasis in platelet-related diseases. Int J Mol Sci 20:5258
Olechnowicz J, Tinkov A, Skalny A, Suliburska J (2018) Zinc status is associated with inflammation, oxidative stress, lipid, and glucose metabolism. J Physiol Sci 68:19–31
Pilz S, Dobnig H, Winklhofer-Roob BM, Renner W, Seelhorst U, Wellnitz B, Boehm BO, Marz W (2009) Low serum zinc concentrations predict mortality in patients referred to coronary angiography. Br J Nutr 101:1534–1540
Pirkle JL (2013) Laboratory procedure manual. Zinc, Copper And Selenium ICPDRCMS-3006.7. Centers for Disease Control and Prevention. https://www.cdc.gov/nchs/data/nhanes/nhanes_11_12/CUSEZN_G_met_serum_elements.pdf. Accessed 31 Aug 2021
Prandoni P (2007) Venous thromboembolism and atherosclerosis: is there a link? J Thromb Haemost 5(Suppl 1):270–275
Prandoni P, Bilora F, Marchiori A, Bernardi E, Petrobelli F, Lensing AW, Prins MH, Girolami A (2003) An association between atherosclerosis and venous thrombosis. N Engl J Med 348:1435–1441
Prandoni P, Ghirarduzzi A, Prins MH, Pengo V, Davidson BL, Sorensen H, Pesavento R, Iotti M, Casiglia E, Iliceto S, Pagnan A, Lensing AW (2006) Venous thromboembolism and the risk of subsequent symptomatic atherosclerosis. J Thromb Haemost 4:1891–1896
Quist-Paulsen P, Naess IA, Cannegieter SC, Romundstad PR, Christiansen SC, Rosendaal FR, Hammerstrom J (2010) Arterial cardiovascular risk factors and venous thrombosis: results from a population-based, prospective study (the HUNT 2). Haematologica 95:119–125
Ray JG (2003) Dyslipidemia, statins, and venous thromboembolism: a potential risk factor and a potential treatment. Curr Opin Pulm Med 9:378–384
Reich LM, Folsom AR, Key NS, Boland LL, Heckbert SR, Rosamond WD, Cushman M (2006) Prospective study of subclinical atherosclerosis as a risk factor for venous thromboembolism. J Thromb Haemost 4:1909–1913
Reiterer G, MacDonald R, Browning JD, Morrow J, Matveev SV, Daugherty A, Smart E, Toborek M, Hennig B (2005) Zinc deficiency increases plasma lipids and atherosclerotic markers in LDL-receptor-deficient mice. J Nutr 135:2114–2118
Reunanen A, Knekt P, Marniemi J, Maki J, Maatela J, Aromaa A (1996) Serum calcium, magnesium, copper and zinc and risk of cardiovascular death. Eur J Clin Nutr 50:431–437
Salonen JT, Salonen R, Seppanen K, Kantola M, Suntioinen S, Korpela H (1991) Interactions of serum copper, selenium, and low density lipoprotein cholesterol in atherogenesis. BMJ 302:756–760
Simera I, Moher D, Hoey J, Schulz KF, Altman DG (2010) A catalogue of reporting guidelines for health research. Eur J Clin Invest 40:35–53
Smabrekke B, Rinde LB, Hindberg K, Hald EM, Vik A, Wilsgaard T, Lochen ML, Njolstad I, Mathiesen EB, Hansen JB, Braekkan S (2016) Atherosclerotic risk factors and risk of myocardial infarction and venous thromboembolism; time-fixed versus time-varying analyses. The Tromso Study Plos One 11:e0163242
Soinio M, Marniemi J, Laakso M, Pyorala K, Lehto S, Ronnemaa T (2007) Serum zinc level and coronary heart disease events in patients with type 2 diabetes. Diabetes Care 30:523–528
Tubek S (2007) Role of zinc in regulation of arterial blood pressure and in the etiopathogenesis of arterial hypertension. Biol Trace Elem Res 117:39–51
van der Hagen PB, Folsom AR, Jenny NS, Heckbert SR, O’Meara ES, Reich LM, Rosendaal FR, Cushman M (2006) Subclinical atherosclerosis and the risk of future venous thrombosis in the cardiovascular health study. J Thromb Haemost 4:1903–1908
Vu TT, Fredenburgh JC, Weitz JI (2013) Zinc: an important cofactor in haemostasis and thrombosis. Thromb Haemost 109:421–430
Wattanakit K, Lutsey PL, Bell EJ, Gornik H, Cushman M, Heckbert SR, Rosamond WD, Folsom AR (2012) Association between cardiovascular disease risk factors and occurrence of venous thromboembolism. A time-dependent analysis. Thromb Haemost 108:508–515
Zhu D, Su Y, Zheng Y, Fu B, Tang L, Qin YX (2018) Zinc regulates vascular endothelial cell activity through zinc-sensing receptor ZnR/GPR39. Am J Physiol Cell Physiol 314:C404–C414
Acknowledgements
We thank the staff of the Kuopio Research Institute of Exercise Medicine and the Research Institute of Public Health and University of Eastern Finland, Kuopio, Finland for the data collection in the study.
Funding
J.A.L. acknowledges support from The Finnish Foundation for Cardiovascular Research, Helsinki, Finland.
Author information
Authors and Affiliations
Contributions
SKK conceived and planned the study and methodology, conducted data curation, carried out the statistical analysis and prepared an original draft; SYJ conceived and planned the study; JAL conceived and planned the study and methodology; all authors contributed to writing, reviewing, and editing of the manuscript, provided insights on the topic, discussed the results and critically revised the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare there are no competing interests.
Informed consent
The Research Ethics Committee of the University of Eastern Finland approved the study (reference #:143/97), and each participant gave written informed consent.
Ethical approval
All study procedures were conducted according to the Declaration of Helsinki.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kunutsor, S.K., Jae, S.Y. & Laukkanen, J.A. No evidence of a prospective relationship between serum zinc and venous thromboembolism in Caucasian men: a cohort study. Biometals 35, 785–793 (2022). https://doi.org/10.1007/s10534-022-00402-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-022-00402-8