Abstract
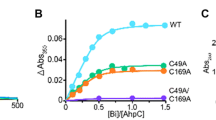
Helicobacter pylori causes various gastric diseases, such as gastritis, peptic ulcerations and gastric cancer. Triple therapy combining bismuth compounds with two antibiotics is the cornerstone of the treatment of H. pylori infections. Up to now, the molecular mechanisms by which bismuth inhibits the growth of H. pylori are far from clear. In the bacterial tricarboxylic acid (TCA) cycle, fumarase catalyses the reversible hydration of fumarate to malic acid. Our previous proteomic work indicated that fumarase was capable of bismuth-binding. The interactions as well as the inhibitory effects of bismuth to fumarase have been characterized in this study. The titration of bismuth showed that each fumarase monomer binds one mol equiv of Bi3+, with negligible secondary structural change. Bismuth-binding results in a near stoichiometric inactivation of the enzyme, leading to an apparent non-competitive mechanism as reflected by the Lineweaver–Burk plots. Our collective data indicate that the TCA cycle is a potential molecular target of bismuth drugs in H. pylori.




Similar content being viewed by others
Abbreviations
- DTNB:
-
5,5′-Dithiobis(2-nitrobenzoic acid)
- FRD:
-
Fumarate reductase
- H. pylori :
-
Helicobacter pylori
- FPLC:
-
Fast protein liquid chromatography
- MS:
-
Mass spectrometer
- PBS:
-
Phosphate-buffered saline
- SDH:
-
Succinate dehydrogenase
- SDS–PAGE:
-
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis
- TCA:
-
Tricarboxylic acid
References
Anil B, Song B, Tang Y, Raleigh DP (2004) Exploiting the right side of the Ramachandran plot: substitution of glycines by d-alanine can significantly increase protein stability. J Am Chem Soc 126:13194–13195
Arcucci A, Montagnani S, Gionti E (2006) Expression and intracellular localization of Pyk2 in normal and v-src transformed chicken epiphyseal chondrocytes. Biochimie 88:77–84
Arnold K, Bordoli L, Kopp J (2006) The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics 22:195–201
Bergmeyer HU, Gawehn K, Grassl M (1974) In: HU Bergmeyer (ed) Methods of enzymatic analysis. Academic Press, New York
Blaser MJ (1987) Gastric Campylobacter-like organisms, gastritis, and peptic ulcer disease. Gastroenterology 93:371–383
Celerier J, Schmid G, Le Caer JP, Gimenez-Roqueplo AP, Bur D, Friedlein A, Langen H, Corvol P, Jeunemaitre X (2000) Characterization of a human angiotensinogen cleaved in its reactive center loop by a proteolytic activity from Chinese hamster ovary cells. J Biol Chem 275:10648–10654
Clauser KR, Baker P, Burlingame AL (1999) Role of accurate mass measurement (±10 ppm) in protein identification strategies employing MS or MS/MS and database searching. Anal Chem 71:2871–2882
Coligan JE, Dunn BM, Ploegh HL, Speicher DW, Wingfield PT (2001) Current protocols in protein science. Wiley, New York
Cun S, Li H, Ge R, Lin MC, Sun H (2008) A histidine-rich and cysteine-rich metal-binding domain at the C terminus of heat shock protein A from Helicobacter pylori: implication for nickel homeostasis and bismuth susceptibility. J Biol Chem 283:15142–15151
DeLano WL (2008) The PyMOL molecular graphics system. DeLano Scientific LLC, Palo Alto
Dundon WG, Polenghi A, Del Guidice G, Rappuoli R, Montecucco C (2001) Neutrophil-activating protein (HP-NAP) versus ferritin (Pfr): comparison of synthesis in Helicobacter pylori. FEMS Microbiol Lett 199:143–149
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77
Ge Z (2002) Potential of fumarate reductase as a novel therapeutic target in Helicobacter pylori infection. Expert Opin Ther Targets 6:135–146
Ge R, Sun H (2007) Bioinorganic chemistry of bismuth and antimony: target sites of metallodrugs. Acc Chem Res 40:267–274
Ge Z, Feng Y, Dangler CA, Xu S, Taylor NS, Fox JG (2000) Fumarate reductase is essential for Helicobacter pylori colonization of the mouse stomach. Microb Pathog 29:279–287
Ge R, Watt RM, Sun X, Tanner JA, He QY, Huang JD, Sun H (2006a) Expression and characterization of a histidine-rich protein, Hpn: potential for Ni2+ storage in Helicobacter pylori. Biochem J 393:285–293
Ge R, Zhang Y, Sun X, Watt RM, He QY, Huang JD, Wilcox DE, Sun H (2006b) Thermodynamic and kinetic aspects of metal binding to the histidine-rich protein, Hpn. J Am Chem Soc 128:11330–11331
Ge R, Sun X, Gu Q, Watt RM, Tanner JA, Wong BC, Xia HH, Huang JD, He QY, Sun H (2007) A proteomic approach for the identification of bismuth-binding proteins in Helicobacter pylori. J Biol Inorg Chem 12:831–842
Guest JR, Miles JS, Roberts RE, Woods SA (1985) The fumarase genes of Escherichia coli: location of the fumB gene and discovery of a new gene (fumC). J Gen Microbiol 131:2971–2984
He QY, Lau GK, Zhou Y, Yuen ST, Lin MC, Kung HF, Chiu JF (2003) Serum biomarkers of hepatitis B virus infected liver inflammation: a proteomic study. Proteomics 3:666–674
Hovmoller S, Zhou T, Ohlson T (2002) Conformations of amino acids in proteins. Acta Crystallogr D 58:768–776
Jin L, Szeto KY, Zhang L, Du W, Sun H (2004) Inhibition of alcohol dehydrogenase by bismuth. J Inorg Biochem 98:1331–1337
Lambert JR, Midolo P (1997) The actions of bismuth in the treatment of Helicobacter pylori infection. Aliment Pharmacol Ther 11:27–33
Maniatis T, Fritsch EF, Sambrook J (1982) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, New York
Megraud F (2004) Basis for the management of drug-resistant Helicobacter pylori infection. Drugs 64:1893–1904
Mendz GL, Hazell SL, Srinivasan S (1995) Fumarate reductase: a target for therapeutic intervention against Helicobacter pylori. Arch Biochem Biophys 321:153–159
Novotny M, Kleywegt GJ (2005) A survey of left-handed helices in protein structures. J Mol Biol 347:231–241
Pitson SM, Mendz GL, Srinivasan S, Hazell SL (1999) The tricarboxylic acid cycle of Helicobacter pylori. Eur J Biochem 260:258–267
Sreerama N, Woody RW (2000) Estimation of protein secondary structure from circular dichroism spectra: comparison of CONTIN, SELCON, and CDSSTR methods with an expanded reference set. Anal Biochem 287:252–260
Sudarshan S, Linehan WM, Neckers L (2007) HIF and fumarate hydratase in renal cancer. Br J Cancer 96:403–407
Sun H, Szeto KY (2003) Binding of bismuth to serum proteins: implication for targets of Bi(III) in blood plasma. J Inorg Biochem 94:114–120
Sun H, Li H, Harvey I, Sadler PJ (1999) Interactions of bismuth complexes with metallothionein(II). J Biol Chem 274:29094–29101
Sun X, Ge R, Chiu JF, Sun H, He QY (2008) Identification of proteins related to nickel homeostasis in Helicobacter pylori by immobilized metal affinity chromatography and two-dimensional gel electrophoresis. Met Based Drugs 2008:289490
Sun X, Ge R, Cai Z, Sun H, He Q-Y (2009) Iron depletion decreases proliferation and induces apoptosis in a human colonic adenocarcinoma cell line, Caco2. J Inorg Biochem 103:1074–1081
Sun X, Ge R, Zhang D, Sun H, He QY (2010) Iron-containing lipoprotein SiaA in SiaABC, the primary heme transporter of Streptococcus pyogenes. J Biol Inorg Chem 15:1265–1273
Tomb JF, White O, Kerlavage AR, Clayton RA, Sutton GG, Fleischmann RD, Ketchum KA, Klenk HP, Gill S, Dougherty BA, Nelson K, Quackenbush J, Zhou L, Kirkness EF, Peterson S, Loftus B, Richardson D, Dodson R, Khalak HG, Glodek A, McKenney K, Fitzegerald LM, Lee N, Adams MD, Hickey EK, Berg DE, Gocayne JD, Utterback TR, Peterson JD, Kelley JM, Cotton MD, Weidman JM, Fujii C, Bowman C, Watthey L, Wallin E, Hayes WS, Borodovsky M, Karp PD, Smith HO, Fraser CM, Venter JC (1997) The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature 388:539–547
van Veen TA, van Rijen HV, Jongsma HJ (2000) Electrical conductance of mouse connexin45 gap junction channels is modulated by phosphorylation. Cardiovasc Res 46:496–510
Weaver T, Banaszak L (1996) Crystallographic studies of the catalytic and a second site in fumarase C from Escherichia coli. Biochemistry 35:13955–13965
Weaver TM, Levitt DG, Donnelly MI, Stevens PP, Banaszak LJ (1995) The multisubunit active site of fumarase C from Escherichia coli. Nat Struct Biol 2:654–662
Woods SA, Schwartzbach SD, Guest JR (1988) Two biochemically distinct classes of fumarase in Escherichia coli. Biochim Biophys Acta 954:14–26
Xia HH, Wong BC, Talley NJ, Lam SK (2002) Alternative and rescue treatment regimens for Helicobacter pylori eradication. Expert Opin Pharmacother 3:1301–1311
Yang Y, Valera VA, Padilla-Nash HM, Sourbier C, Vocke CD, Vira MA, Abu-Asab MS, Bratslavsky G, Tsokos M, Merino MJ, Pinto PA, Srinivasan R, Ried T, Neckers L, Linehan WM (2010) UOK 262 cell line, fumarate hydratase deficient (FH−/FH−) hereditary leiomyomatosis renal cell carcinoma: in vitro and in vivo model of an aberrant energy metabolic pathway in human cancer. Cancer Genet Cytogenet 196:45–55
Yogev O, Singer E, Shaulian E, Goldberg M, Fox TD, Pines O (2010) Fumarase: a mitochondrial metabolic enzyme and a cytosolic/nuclear component of the DNA damage response. PLoS Biol 8:e1000328
Zhang L, Mulrooney SB, Leung FK, Zeng YB, Ko BBC, Hausinger RP, Sun H (2006) Inhibition of urease by bismuth(III): implications for the mechanisms of action of bismuth drugs. Biometals 19:503–511
Acknowledgments
The authors thank the anonymous reviewer for insightful comments. This work was supported by National Natural Science Foundation of China (20801061), Guangdong Natural Science Foundation (8451027501001233) and the Fundamental Research Funds for the Central Universities (10lgpy19).
Author information
Authors and Affiliations
Corresponding author
Additional information
Zhuo Chen and Qinglu Zhou contribute equally to this work.
Rights and permissions
About this article
Cite this article
Chen, Z., Zhou, Q. & Ge, R. Inhibition of fumarase by bismuth(III): implications for the tricarboxylic acid cycle as a potential target of bismuth drugs in Helicobacter pylori . Biometals 25, 95–102 (2012). https://doi.org/10.1007/s10534-011-9485-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-011-9485-7