Abstract
Carbon cycles in coastal waters are highly sensitive to human activities and play important roles in global carbon budgets. CO2 sink–source behavior is regulated by spatiotemporal variations in net biological productivity, but the contribution of macrophyte habitats including macroalgae aquaculture to atmospheric CO2 removal has not been well quantified. We investigated the variations in the carbonate system and dissolved organic carbon (DOC) in human-impacted macrophyte habitats and analyzed the biogeochemical drivers for the variations of these processes. Cultivated macroalgal metabolism (photosynthesis, respiration, calcification, and DOC release) was quantified by in situ field-bag experiments. Cultivated macroalgae took up dissolved inorganic carbon (DIC) (16.2–439 mmol-C m−2 day−1) and released DOC (1.2–146 mmol-C m−2 day−1). We estimated that seagrass beds and macroalgae farming contributed 0.8 and 0.4 mmol-C m−2 day−1 of the in situ total CO2 removal (5.7 and 6.7 mmol-C m−2 day−1, respectively) during their growing period in a semi-enclosed embayment but efficient water exchange (i.e., short residence time) in an open coastal area precluded detection of the contribution of macrophyte habitats to the CO2 removal. Although hydrological processes, biological metabolism, and organic carbon storage processes would contribute to the net CO2 sink–source behavior, our analyses distinguished the contribution of macrophytes from other factors. Our findings imply that macroalgae farming, in addition to restoring and creating macrophyte habitats, has potential for atmospheric CO2 removal.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coastal waters play major roles in global carbon cycles and budgets because they connect terrestrial with oceanic systems; they are also impacted by human activities (Bauer et al. 2013; Regnier et al. 2013; Friedlingstein et al. 2019). Despite the importance of coastal waters, the sources and sinks for atmospheric carbon dioxide (CO2) in these systems remain uncertain because of their diverse and dynamic physical and biogeochemical processes (Kuwae et al. 2019; Roobaert et al. 2019). Estuaries, the coastal waters most affected by riverine inputs, are known to be global net CO2 sources (0.10–0.27 Pg C year−1, Laruelle et al. 2010; Cai 2011; Chen et al. 2013); in contrast, continental shelves act as global net CO2 sinks (− 0.40 to − 0.20 Pg C year−1, Laruelle et al. 2010; Cai 2011; Chen et al. 2013; Roobaert et al. 2019). The uncertainties in these estimates derive from spatial and temporal biases in data availability (Laruelle et al. 2017).
CO2 emissions from estuaries are globally significant because of the inputs of CO2-enriched freshwater, as well as heterotrophic generation of CO2 from allochthonous and autochthonous organic carbon (Cai 2011; Regnier et al. 2013; Yao et al. 2020). However, recent studies have shown that coastal waters can function as net sinks for atmospheric CO2, depending on net ecosystem productivity (Cotovicz et al. 2021; Maher and Eyre 2012; Tokoro et al. 2019), freshwater influence (Jiang et al. 2008; Koné et al. 2009; Kubo et al. 2017), and the balance of carbon and nutrient loads (Kuwae et al. 2016). In particular, coastal waters functioning as net CO2 sinks are often strongly impacted by human activities such as urbanization and agriculture (Cotovicz et al. 2015; Kubo et al. 2017; Kuwae et al. 2019). Nutrient loads from cities, agriculture, and livestock rearing can promote primary production and autotrophy, lowering the partial pressure of CO2 (pCO2) and CO2 efflux rates in estuarine waters. For example, the historical increase in riverine loads of dissolved inorganic nitrogen (DIN) associated with agricultural runoff and land-use changes has substantially enhanced net ecosystem production, which can switch coastal systems from being net sources of CO2 to sinks (Chou et al. 2013; St-Laurent et al. 2020).
Human activities alter the distribution and productivity of marine macrophytes, which potentially regulate carbon cycles and budgets in coastal waters. Aquaculture of marine macroalgae (i.e., seaweeds) is a direct human intervention to enhance production. Because macroalgae would be a significant contributor to sequestration of carbon in the ocean (Krause-Jensen and Duarte 2016), interest in using macroalgae aquaculture to mitigate climate change is increasing globally (Duarte et al. 2022; Froehlich et al. 2019; Wu et al. 2020). The construction of coastal infrastructure (e.g., seawalls and breakwaters) provides substrata for colonization and growth of macroalgae, which may facilitate carbon sequestration (Dafforn et al. 2015; Kuwae and Crooks 2021). The conservation, restoration, and creation of vegetated coastal ecosystems are expected as active interventions to maintain or increase carbon sinks (Griscom et al. 2017; Kuwae et al. 2022; Macreadie et al. 2019). These human interventions would be substantial in coastal waters close to large cities.
Vegetated coastal ecosystems function as the most active reservoirs of organic carbon among aquatic systems and store large amounts of organic carbon in their sediments (McLeod et al. 2011). However, it remains uncertain whether these ecosystems and adjoining waters serve as net sinks or sources of atmospheric CO2 (Kuwae et al. 2019; Macreadie et al. 2019). Recent studies have demonstrated that submerged macrophyte habitats, such as macroalgae and seagrass beds, and macroalgae farming, can act as net CO2 sinks because of their high primary productivity (Jiang et al. 2013; Han et al. 2017; Maher and Eyre 2012; Tokoro et al. 2014; Watanabe et al. 2020). Tokoro et al. (2014) showed that the net ecosystem production of seagrass meadows changes the carbonate chemistry and can lower pCO2 below atmospheric equilibrium. However, the autotrophy of marine macrophyte habitats is not necessarily a sufficient condition for net CO2 sink. Hydrological processes (e.g., freshwater–seawater mixing and water exchanges) and organic carbon storage processes (e.g., sequestration in the seafloor and water column) would contribute to the net CO2 sink behavior (Kuwae et al. 2016). A substantial amount of carbon assimilated by submerged macrophytes is exported to outside their habitats as dissolved organic carbon (DOC) (Duarte and Krause-Jensen 2017; Krause-Jensen and Duarte 2016). A part of macrophyte DOC is recalcitrant and contributes to long-term carbon sequestration in the water column (Li et al. 2022; Watanabe et al. 2020).
Macroalgae and seagrasses lower pCO2 and increase DOC in their habitats and adjoining waters, which are key processes in removal of atmospheric CO2 (Macreadie et al. 2019; Watanabe and Kuwae 2015). However, the variabilities of pCO2 and DOC in coastal waters are affected by complex processes, including hydrological dynamics and phytoplankton metabolism. Hence the contribution of submerged macrophytes to the variabilities of pCO2 and DOC has not been quantified sufficiently. Statistical analyses would help to distinguish the effects of the macrophytes on pCO2 and DOC from those of other biogeochemical drivers. To quantify the contribution of marine macrophytes to atmospheric CO2 removal, it is essential to establish a plausible baseline for assessing the effects of interventions into marine macrophyte habitats for carbon credit projects and GHG inventories.
In this study, we examined spatial and temporal variations in pCO2, other carbonate chemistry variables, DOC, and physical and biogeochemical factors in human-impacted coastal waters. To quantify the contribution of macrophyte habitats (i.e., macroalgae farms, and natural seagrass and macroalgae beds) on pCO2 and DOC variations in coastal waters, we statistically analyzed the drivers determining temporal and spatial variations in pCO2 and DOC by comparing vegetated habitats with non-vegetated areas. We hypothesized that marine macrophytes substantially affect the spatial variation of pCO2 and DOC during their growth period. In addition, we hypothesized that pCO2 and DOC variations were regulated by the productivity of phytoplankton and macrophyte and freshwater-seawater mixing in coastal waters over annual timescales.
Materials and methods
Study site
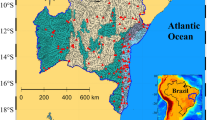
The present study was conducted in the coastal waters of Kanazawa Bay (35° 20′ 1.6″ N, 139° 38′ 34.0″ E) and those off the coast of Hannan (34° 21′ 24.37″ N, 135° 13′ 17.21″ E) (Fig. 1). Kanazawa Bay is a semi-enclosed embayment close to the mouth of Tokyo Bay, along the northwestern Pacific (Fig. 1). The embayment is located in the southern part of Yokohama city (population: approximately 3.7 million in 2015); the bay is impacted by human activities along the coast of Tokyo Bay. The majority of the coastline in Kanazawa Bay is seawalls, and an artificial island is located in the center of the embayment. The depths of the entrance, the center, and the shallow inner zone are 20 m, 7 m, and ∼ 2 m, respectively. Kanazawa Bay receives freshwater inputs from three rivers that flow into Hiragata Bay and the two waterways that connect Hiragata Bay to Kanazawa Bay (Fig. 1). In addition, freshwater discharged from rivers flowing into the inner parts of Tokyo Bay influence the study site when the river discharges are high.
Maps of Kanazawa Bay and off the coast of Hannan showing the locations of sampling stations: KZ1–4 and HN1–6 are macroalgae farms, KZ5–9 and HN7–8 are seagrass beds, KZ10–11 are in the waterways connecting the bay to the adjoining tidal flat (Hiragata Bay), and KZ12–13 and HN9–10 are offshore from the habitats. Blue and green colors represent macroalgae farms and seagrass beds, respectively. The locations of airports (Tokyo International Airport, HND; Kansai International Airport, KIX) are shown
The coast of Hannan is an open coastline facing Osaka Bay, along the northwestern Pacific (Fig. 1). The coastal water of Osaka Bay is affected by the human activities of Osaka city (population: approximately 2.7 million in 2015) and its surrounding area. The beach on the coast of Hannan is sandy with some manmade structures. The depths of the study sites range from 1 to 15 m. Tidal currents flow parallel to the shore.
Suspended aquaculture of macroalgae is practiced at both sites (Fig. 1). In Kanazawa Bay, the cultivated areas of the brown macroalgae Undaria pinnatifida (wakame) and Saccharina japonica (kombu) are 2.0 and 0.4 ha, respectively. In the coastal waters off the coast of Hannan, U. pinnatifida and the red macroalga Neopyropia yezoensis (nori) are cultivated on 4.2 and 1.9 ha, respectively. The cultivation periods of these species are from November to April (Fig. S1).
Seagrass meadows dominated by Zostera marina occupy parts of the inner zones (depths shallower than 5 m) of Kanazawa Bay and the Hannan coast (Fig. 1). The northern seagrass meadow in the shallow zone of Kanazawa Bay (the beach of Sea Park Yokohama) was created by reclamation (Kuwae et al. 2022). Citizens are engaged in seagrass bed restoration activities along the coast of Hannan (Fig. 1). Artificial seawalls and reefs are naturally inhabited by macroalgae species such as U. pinnatifida and Sargassum horneri in Kanazawa Bay (Fig. S1; Appendix S1), and Ecklonia spp., U. pinnatifida, and Sargassum spp. off the coast of Hannan (Yoneda et al. 2014).
Water sampling surveys
In Kanazawa Bay, surveys were conducted at least monthly from October 2017 to March 2019, with the exception of April–May 2018 and November–December 2018, because of difficulties in arranging a survey vessel. To compare the effects of macroalgae aquaculture on pCO2 and DOC variations in Kanazawa Bay with those in a system with different hydrographic characteristics, water sampling was conducted only during the macroalgae cultivation periods in March 2020 and January and February 2021 in the coastal waters off the coast of Hannan (Fig. S1). To determine the effects of marine macrophytes, the study stations were divided according to the habitat types into MA (macroalgae farm area: KZ1–4 in Kanazawa Bay and HN1–6 on the coast of Hannan), SG (seagrass bed area: KZ5–9 and HN7–8), TF (tidal flat area: KZ10–11), and OUT (offshore of the habitats: KZ12–13 and HN9–10) (Fig. 1). The OUT stations, where no macroalgae inhabited, were chosen as reference stations. Macroalgae on the revetments are widely distributed in these sites and would partially contribute to the results of MA, SG, and TF stations. Although natural macroalgae and seagrasses inhabit the coasts of our sites, they accounted for about 1 and 0.02% of the total area of Tokyo Bay and Osaka Bay in 1990s, respectively (Ministry of the Environment 1996). Because the total area of these habitats in Japan is still thought to be decreasing (Terada et al. 2021), it is unlikely that the area in our study sites has increased compared to the 1990s. Therefore, the impact of natural macroalgae and seagrass habitats on the waters of OUT stations, which are approximately 1 km away from the shores, can be assumed to be negligible.
During the surveys, surface and bottom water samples for analyses of dissolved inorganic carbon (DIC), total alkalinity (TAlk), and dissolved organic carbon (DOC) were collected from a research vessel twice (morning and evening) during the daytime at the stations of Kanazawa Bay. Because Kanazawa Bay was seasonally stratified, bottom water samples were collected from 1 m above the bottom, except at stations shallower than 2 m. Surface water samples were collected from a research vessel 3 times (morning, noon, and evening) during the daytime at the stations off the Hannan coast because no stratification developed during the observation periods.
Samples for DIC and TAlk were dispensed into 250-mL Schott Duran bottles and preserved with saturated mercuric chloride solutions (200 μL per bottle). Water samples for DOC analysis were filtered through 0.2-μm pore size polytetrafluoroethylene filters (DISMIC–25HP; Advantec, Durham, NC, USA) into precombusted (450 °C for 2 h) 50-mL glass vials. DOC samples were frozen at − 20 °C until analysis. At each station, salinity, temperature, and chlorophyll fluorescence were recorded with either a RINKO-Profiler (ASTD102, JFE Advantech, Nishinomiya, Japan) or conductivity–temperature (INFINITY-CT, JFE Advantech) and fluorescence (COMPACT-CLW, JFE Advantech) sensors. We calculated chlorophyll a (Chl. a) concentrations using the regression line between fluorescence sensor data and spectrophotometrically measured Chl. a data.
Field-bag experiments
Field-bag experiments (e.g., Wada et al. 2007; Watanabe et al. 2020) were conducted in situ to quantify the carbon metabolism of cultivated macroalgae twice for each species during the winter cultivation period in 2021 at both sites. By conducting two experiments at each site, we attempted to reduce uncertainty due to the different times of the experiments as much as possible (Watanabe et al. 2020). We measured the metabolism of U. pinnatifida and S. japonica in Kanazawa Bay, and U. pinnatifida and N. yezoensis off the coast of Hannan. The experiments were conducted using whole detached thalli, which were kept in ambient seawater for > 1 h for acclimation before the experiments. A previous study has shown that the detachment procedure does not affect DOC release from the thalli (Weigel and Pfister 2021).
The thallus of each macroalga was placed in a plastic bag containing ambient seawater. The volume of seawater and the wet weight of the thallus were recorded for each bag. The open end of the bag was tightly tied to prevent air from entering. Triplicate transparent and dark bags were set up to measure the changes of DIC, TAlk, and DOC due to macroalgal metabolism (Watanabe et al. 2020). To assess the contribution of phytoplankton, an otherwise identical set of transparent and dark bags were filled only with ambient seawater as control bags. Water samples were collected from the bags at the start of the experiment and about 4 h later. Because the detachment of thalli from the substrata and the alteration of the conditions around them may stress the thalli (Frontier et al. 2021), we implemented an experimental design that minimized incubation time (i.e., 4 h) and avoided taking the thalli out from the sea surface to reduce such stresses as much as possible.
The collected water samples for carbonate chemistry and DOC analyses were preserved as described above. The thalli in three bags were immediately transported to the laboratory, weighed, dried (60 °C for more than 24 h) until they reached constant weight, and then weighed again to obtain the water content.
The biomass of cultivated macroalgae in each field bag experiment was measured. For macroalgae cultivated on longlines (i.e., U. pinnatifida and S. japonica), all thalli attached to several meters of rope were collected, and the total wet weight was measured. The biomass per area was calculated taking into account the length, number, and spacing of the ropes. Because N. yezoensis was cultivated on nets, the biomass per unit area was measured by using triplicate quadrats (0.25 m2).
Gross community production (GCP), community respiration (CR), net community production (NCP), community calcification (CC), and DOC release were determined from the changes in DIC, TAlk, and DOC of the field-bag experiments. These metabolic variables were calculated from the equations proposed by Watanabe et al. (2020). The daily rates of metabolic parameters were calculated by using the mean macroalgal biomass, the lengths of the photoperiods, and the hourly metabolic rates obtained from the transparent and dark bag experiments, respectively. The photoperiod was defined as the time interval between sunrise and sunset; photoperiods were obtained from Automated Meteorological Data Acquisition System (AMeDAS) data provided by the Japan Meteorological Agency (available at https://www.jma.go.jp, last access: 15 March 2021).
Chemical analyses
DIC and TAlk were analyzed with a titration method by batch-sample analyzer (ATT-05 and ATT-15; Kimoto Electric, Osaka, Japan). The analytical precision of the system, based on the standard deviation of multiple reference replicates, was normally within ± 2 μmol L−1 for both DIC and TAlk. The value of pCO2 in seawater was estimated by the CO2SYS program (Lewis et al. 1998) from the TAlk, DIC, salinity, and water temperature of each sample (Zeebe and Wolf-Gladrow 2001). We used formulations for the dissociation constants of carbonic acid from Dickson and Millero (1987) and the solubility coefficient of CO2 from Weiss (1974).
DOC was measured at least in triplicate with a total organic carbon analyzer (TOC-L; Shimadzu, Kyoto, Japan) as non-purgeable organic carbon. Potassium hydrogen phthalate (Wako Pure Industries, Osaka, Japan) adjusted to three concentrations (approximately 83, 166, and 332 μM) was used as the calibration standard. The coefficient of variation of the triplicate analyses was less than 2%.
Air–water CO2 flux calculation
The bulk formula approach was used to estimate the air–water CO2 flux (\({\text{F}}_{{{\text{CO}}_{{2}} }}\)) as follows:
where pCO2water and pCO2air are the pCO2 in the seawater and the atmosphere, respectively. \({\text{F}}_{{{\text{CO}}_{{2}} }}\) values were calculated for all surface water data. We used the annual averaged pCO2air (411 ± 14 μatm in Tokyo Bay; 413 ± 13 μatm in Osaka Bay) obtained from the Surface Ocean CO2 Atlas (Bakker et al. 2016). K0 is the CO2 solubility in the water (Weiss 1974). A negative \({\text{F}}_{{{\text{CO}}_{{2}} }}\) value indicates CO2 uptake from the air to the seawater. The gas transfer velocity (k) was estimated from empirical relationships between k and the wind speed above the surface of the water. In this study, to account for uncertainty due to parameter selection for k, we used two parameterizations for estuaries (Jiang et al. 2008, J08; Raymond and Cole 2001, RC01) and two for oceanic waters (McGillis et al. 2001, M01; Wanninkhof 2014, W14) as follows:
where the Schmidt number (Sc) was determined from the water temperature and salinity of the water surface (Wanninkhof 2014) and U10 represents the wind speed at a height of 10 m above the water surface. We determined U10 by assuming that there was a logarithmic relationship between wind speed, height, and the roughness of the surface (Kondo 2000). Wind speed was obtained from AMeDAS data (https://www.jma.go.jp) measured at Yokohama and Kankujima for Kanazawa Bay and the coast of Hannan, respectively, and provided by the Japan Meteorological Agency. For wind speed corrections, we used the roughness data at the observation sites, which were estimated from land use information (Kuwagata and Kondo 1990).
Statistical analyses
We used R statistical packages (R Core Team 2022) to examine the effects of primary producers and other biogeochemical factors on the variations in pCO2 and DOC with generalized linear models (GLMs). The GLMs were constructed separately for an annual-scale model and seasonal-scale models. We chose a GLM structure with a gamma distribution and log link because dependent variables were non-negative continuous variables. A priori selection of candidate models was based on the principle of parsimony and scientific plausibility (Burnham and Anderson 2002). The best model was selected according to the Akaike information criterion (AIC).
We used the dataset of Kanazawa Bay for 6 time periods (Table S2): Annual (October 2017–September 2018), February–March (2018 and 2019), June–July (2018), August–September (2018), October–November (2017 and 2018), and December–January (2017, 2018, and 2019). With the dataset of Hannan, we constructed a model for January–March (2020 and 2021) (Table S2). Although the years are different for the datasets of Kanazawa Bay and the Hannan coast, both sites have statistical analyses of results from multiple years. Because the results of two sites were compared as multi-year averages, the bias of different year sampling could be minimized as much as possible.
pCO2 and DOC were the dependent variables. Salinity, water temperature, Chl. a, and habitat type (i.e., MA, SG, TF, and OUT) were the explanatory variables. In this study, the non-existence of collinearity among all explanatory variable was ensured by checking that the variance inflation factors (VIF) were lower than 5. Hierarchical partitioning was used to estimate relative importance of an explanatory variable by computing goodness of fit of all GLMs (Murray and Conner 2009). As the goodness of fit measure, we used root mean square prediction error.
Results
Carbonate chemistry, DOC, and biogeochemical drivers
Average surface pCO2 values of all stations in Kanazawa Bay were lower than atmospheric equilibrium (ca. 411 µatm) except for two sampling surveys (Fig. 2a). Surface pCO2 increased when surface salinity decreased because of river discharge (Fig. 2g). Surface and bottom pCO2 values diverged during summer corresponding to the formation of a thermocline (Fig. 2h). pCO2 tended to be low while the Chl. a concentration was increasing (Fig. 2i). DOC concentrations increased from spring to summer, closely related to the increase in temperature (Fig. 2b, h). TAlk and DIC concentrations tended to decrease with decreasing salinity in the surface water (Fig. 2c, d, g). The decrease in DIC was also linked to the increase in Chl. a. Spatially averaged \({\text{F}}_{{{\text{CO}}_{{2}} }}\), which was controlled by surface pCO2 and wind velocity, varied seasonally (Fig. 2a, e, f). Spatially averaged \({\text{F}}_{{{\text{CO}}_{{2}} }}\) was low and indicated a strong influx of CO2 from winter to spring in Kanazawa Bay (Fig. 2e). In contrast, spatially averaged \({\text{F}}_{{{\text{CO}}_{{2}} }}\) became positive (i.e., CO2 efflux) when surface salinity became low because of river discharge (Fig. 2e, g). Spatially averaged \({\text{F}}_{{{\text{CO}}_{{2}} }}\) varied over time from − 13.7 to 7.1 mmol m−2 day−1. Annual mean \({\text{F}}_{{{\text{CO}}_{{2}} }}\) was negative (− 3.4 mmol m−2 day−1; range: − 4.2 to − 2.1 mmol m−2 day−1), indicating that Kanazawa Bay acted as a net CO2 sink. Low-pCO2 conditions (i.e., the strong influx of atmospheric CO2) corresponded with the increase of TAlk/DIC ratios (Fig. 3).
Seasonal variations in the spatial averages of partial pressure of CO2 (pCO2) (a), dissolved organic carbon (DOC) (b), dissolved inorganic carbon (DIC) (c), total alkalinity (TAlk) (d), air–water CO2 fluxes (\({\text{F}}_{{{\text{CO}}_{{2}} }}\)) (e), wind speed (U10) (f), salinity (g), temperature (h), and chlorophyll a (Chl. a) (i) across all stations in Kanazawa Bay. Error bars represent standard deviations. The solid line and shaded area of \({\text{F}}_{{{\text{CO}}_{{2}} }}\) indicate the average value and the range of estimated \({\text{F}}_{{{\text{CO}}_{{2}} }}\) for four gas transfer velocity parameters
Off the coast of Hannan in March 2020 and January and February 2021, all surface water samples were undersaturated with respect to atmospheric CO2 (ca. 413 µatm) (Table 1 and Fig. 3). As in Kanazawa Bay, pCO2 values off Hannan decreased with decreasing DIC/TAlk ratios (Fig. 3). Average \({\text{F}}_{{{\text{CO}}_{{2}} }}\) across all stations ranged from − 16.5 to − 5.3 mmol m−2 day−1 (Table 1). Average pCO2 was lowest in January 2021 when average Chl. a was highest. Salinity and temperature varied little among the three sampling surveys in March 2020 and January and February 2021 off the coast of Hannan in Osaka Bay (Table 1).
Generalized linear models
Annual- and seasonal-scale GLMs supported the effects on pCO2 of salinity, temperature, Chl. a, and habitat types. Habitat types SG, MA, and TF had significant negative effects on pCO2 during the February to March period in Kanazawa Bay but not off the Hannan coast (Table 2). During the February to March period in Kanazawa Bay, the GLM estimated that pCO2 was reduced by an average of 8.8%, 4.5%, and 5.8% at the SG, MA, and TF stations, respectively (Fig. 4a). These percentages at SG and MA stations in Kanazawa Bay were equivalent to 0.8 and 0.4 mmol-C m−2 day−1 of the total removal rate of atmospheric CO2 (5.7 and 6.7 mmol-C m−2 day−1; Fig. 2e) during the February to March period, respectively. TF had significant positive effects on pCO2 during summer (June–September). On an annual scale in Kanazawa Bay, TF stations had significant, positive effects on pCO2. In Kanazawa Bay, the results of the annual model suggested negative effects of salinity and Chl. a and a positive effect of temperature on pCO2 variations (Table 2). The results of the seasonal-scale models suggested that Chl. a had significant negative effects on pCO2 during every period at both sites (Table 2) and that the independent contribution of Chl. a to pCO2 variations over an annual timescale was 71% (Fig. 5a).
Percent changes in pCO2 (a) and DOC (b) relative to those of the water outside of Kanazawa Bay. Percentage change in each carbon variable due to the consideration of habitat type coefficients in each full GLM model are shown. Error bars show 95% confidence intervals (CIs). When the 95% CIs do not overlap with 0, the effect of each habitat type is significant at the 5% level. KZ, Kanazawa Bay; HN, off the coast of Hannan
DOC concentrations were significantly and positively affected by the three habitat types during the February-to-March period in Kanazawa Bay but not off the Hannan coast (Table 2). DOC concentrations during the February to March period in Kanazawa Bay were estimated to increase by an average of 6.2%, 5.1%, and 5.2% at the SG, MA, and TF stations, respectively (Fig. 4b). Temperature and salinity together explained 91% of annual DOC variations (Fig. 5b). In particular, the results of the annual model suggest that DOC concentrations were higher at higher water temperature and lower salinity (Table 2).
Metabolism of cultivated macroalgae
The metabolism variables of cultivated macroalgae were calculated from the results of the field-bag experiments (Tables 3 and S3). The NCP values of macroalgae were positive during the cultivation periods at both sites: 119–264 mmol-C m−2 day−1, 16.2–73.9 mmol-C m−2 day−1, and 433–439 mmol-C m−2 day−1 for U. pinnatifida, S. japonica, and P. yezoensis, respectively. These NCP values implied daily removals of DIC from the water column that were much larger than the estimated removal rates of atmospheric CO2 of 0.8 and 0.4 mmol-C m−2 day−1 by seagrass and macroalgae, respectively. The CC values were about an order of magnitude lower than the NCP values (Table 3). The DOC release rates greatly varied from 1.2 to 146 mmol-C m−2 day−1, depending mainly on the biomass density and cultivated species. DOC release accounted for 31–47% of NCP for U. pinnatifida, 7–12% for S. japonica, and 27–34% for P. yezoensis.
Discussion
Contribution of marine macrophytes to pCO2 and DOC dynamics
The NCP associated with macroalgae farming (Han et al. 2017), seagrass beds (Tokoro et al. 2014; Yates et al. 2007), and macroalgae beds (Ikawa and Oechel 2015; Watanabe et al. 2020) can enhance atmospheric CO2 removal. However, the extent to which macrophytes substantially control pCO2water and \({\text{F}}_{{{\text{CO}}_{{2}} }}\) has not been well quantified. As our GLM results indicate, the growing period of seaweeds and seagrasses (January to March; Fig. S1) is suitable for evaluation of the effects of these macrophytes on coastal carbon cycles. Our results demonstrated that both seagrass beds and macroalgae farms could significantly enhance atmospheric CO2 removal, especially during their growing period in Kanazawa Bay, a semi-enclosed embayment. The removal rates of atmospheric CO2 by seagrass and macroalgae were not equivalent to the removal rates of DIC (i.e., NCP) over daily timescales. In contrast, the enhancement of CO2 removal was not statistically supported off the coast of Hannan, an open coastal system. These findings suggest that the magnitude of DIC uptake by macrophytes does not equate to direct atmospheric CO2 removal on site over timescales from days to a month. In Kanazawa Bay, the metabolism of cultivated macroalgae and the pCO2 and DOC variabilities were measured in different years, which limited the quantitative comparisons of these indices. However, since values of similar order of magnitude were obtained from two field-bag experiments under different biotic and environmental conditions (i.e., different months) (Table 3), comparisons on an order of magnitude scale should be possible.
The inconsistency between DIC uptake by macrophytes and direct atmospheric CO2 removal over timescales from days to a month is a result of re-equilibration of CO2 between the atmosphere and the low-pCO2 water affected by macrophytes, which is slower than macrophyte metabolism (Bach et al. 2023; Hurd et al. 2022). The lateral and vertical transport of macrophyte-affected water dilutes the effect of CO2 removal (Watanabe et al. 2020). These processes spread the footprint of macrophyte carbon uptake over space and time. Technological developments in tracing and modelling the spatial and temporal footprint of macrophyte effects with high-resolution sensors will help to quantify the substantial impact of marine macrophytes on atmospheric CO2 removal (Ross et al. 2023).
The results of seasonal-scale GLMs suggest that the presence of seagrass beds and macroalgae farms significantly lowers seawater pCO2 and increases DOC compared with water outside of Kanazawa Bay during the period from February to March (Fig. 4). Specifically, the autotrophic activity of marine macrophytes takes up DIC and converts it to organic carbon (OC), which lowers seawater pCO2 and releases DOC at bay scale. The results of community metabolism measurements also demonstrate that cultivated macroalgae act as net DIC absorbers and net DOC releasers during the productive period (Table 3). In contrast, such autotrophic effects of these habitats on the pCO2 and DOC were not detected during the non-cultivation period of macroalgae farming and the low growth period of natural seagrass beds (Fig. 4). During summer, OC decomposition stimulated by high water temperature would balance or exceed the NCP of macrophytes, increasing pCO2 in these habitats. The annual model showed no significant net effect of macroalgae habitat on pCO2 (95% CI − 5.7 to + 5.9%; Table 2 and Fig. 4). Because cultivated macroalgae are annual species (Fig. S1), the autotrophic effect of these species would be limited to their growth periods. In contrast, seagrass beds tended to have autotrophic effects on pCO2 (95% CI − 10.7 to + 0.3%; Fig. 4) in the annual-scale GLM, which may be due to the fact that the perennial seagrass community in this region maintains its biomass throughout the year (Fig. S1). Tokoro et al. (2014) also reported that boreal seagrass beds act as annual net CO2 sinks, even though they become heterotrophic during the period of low growth. Also, the positive estimates during July to September had large estimation errors and no significant effect, and thus they contributed less to the annual estimates than the negative estimates during February to March.
We attribute the significant pCO2 increase at TF stations to the enhancement of benthic respiration and decomposition of sedimentary OC during summer (Fig. 4). In contrast, from February to March, the TF stations were autotrophic, which suggests that benthic microalgal production at that time may outweigh the effects of respiration and decomposition (Lin et al. 2021; Otani and Endo 2019). Also, the carbon fluxes of tidal flats are strongly affected by the intrusion of outside waters (Tokoro and Kuwae 2022). The advection of water influenced by macroalgae farming as well as seagrass and macroalgae beds would lower pCO2 and raise DOC, even in tidal flat stations located in the inner part of the bay.
The macroalgae farms off the coast of Hannan act as an autotrophic system, which reduces DIC and increases DOC in the water column. NCP, DOC release rates, and the areal extent of macroalgae farms off the coast of Hannan were larger on average than those in Kanazawa Bay (Tables 3 and S3); however, the GLMs did not support the effects of macroalgae farms and seagrass beds at Hannan on the variations of seawater pCO2 and DOC even during their growth period (Table 2). Water exchange between the habitats and the offshore dilute the water affected by macrophytes (Koweek et al. 2017), which is advected away (Watanabe et al. 2020). The possibility to detect changes in water characteristics modified by macrophytes depends on the carbon metabolism and water exchange rates (i.e., residence time). Surface current velocities in Kanazawa Bay are about 6 m min−1 (Inagaki et al. 1997) and those off the coast of Hannan are 18 m min−1 (Yoneda et al. 2014); thus, there was a substantial difference in residence time. The macrophyte habitats in Kanazawa Bay are located in the inner bay, whereas those off the Hannan coast are located in the channel; thus, low-pCO2 and high-DOC water affected by macrophytes at Hannan would be quickly advected out of the system. Seasonal studies of the diffusion and advection of macrophyte-affected water would be necessary to quantify the impact on the carbon budget, as residence time is seasonally variable.
In this study, we showed that macroalgae released substantial amounts of DOC, and macroalgae farms and seagrass beds raised DOC concentrations at bay scale when they were growing. DOC release rates and DOC/NCP ratios of the cultivated macroalgae were within the ranges of these values reported in previous studies (Pessarrodona et al. 2022; Weigel and Pfister 2021). Macrophyte DOC would be laterally transported away from the habitats. Some macrophyte DOC is recalcitrant, which would contribute to long-term carbon sequestration in the water column (Buck-Wiese et al. 2022; Li et al. 2022; Watanabe et al. 2020). Quantifying and tracing refractory DOC derived from macrophytes in the ocean is a research gap in our understanding of their carbon sequestration functions (Pessarrodona et al. 2023). Future studies should quantify and integrate carbon budgets, including NCP, carbon storage processes (e.g., the release of refractory DOC and OC burial), and the removal of atmospheric CO2 over extensive spatiotemporal scales.
Biogeochemical drivers of pCO2 and DOC variations
Our findings demonstrate that in the coastal waters we studied over annual timescales, spatial and temporal variations in pCO2 are primarily regulated by phytoplankton productivity (for which Chl. a is a proxy), then by freshwater-seawater mixing process (for which salinity and temperature are proxies), and seasonally by macrophyte productivity (Table 2; Fig. 5). This result is consistent with previous reports that pCO2 variability is mainly controlled by phytoplankton productivity in human-impacted coastal areas with abundant nutrients (Cotovicz et al. 2015; Kubo et al. 2017; St-Laurent et al. 2020). Assuming average water temperature (15.9 °C) and salinity (30.8), our GLM showed that CO2 in water in Kanazawa Bay is undersaturated with respect to the atmosphere even under conditions in which Chl. a is close to zero. The offshore water (i.e., northwestern Pacific) has been reported to be one of the largest CO2 sinks in the world (Takahashi et al. 2009; Tokoro et al. 2021), which is consistent with the CO2 sink behavior of the marine-dominated bay.
Annual DOC variations in Kanazawa Bay were primarily controlled by water temperature, then by phytoplankton and salinity, and seasonally macrophytes (Table 2; Fig. 5). The strong relationship between DOC and water temperature would support the major contribution of DOC supplied from offshore (Kubo et al. 2015). Primary producers including phytoplankton and marine macrophytes convert DIC into OC via photosynthesis, subsequently releasing a part of the assimilated OC as DOC to the water column (Baines and Pace 1991; Barrón and Duarte 2009). Kanazawa Bay is an annual net autotrophic system, indicating that the bay plays a role as a net DOC supplier to the water column.
We demonstrated that the variations in carbon variables are statistically predictable using GLMs with the environmental variables Chl. a, salinity, temperature, and habitat type. Such simple statistical models can help upscaling of carbon distribution maps to fill gaps in spatial and temporal coverage and improve the estimation of global carbon budgets (Lauerwald et al. 2015).
Air–water CO2 flux over annual timescales
Estuaries are affected by substantial amounts of riverine carbon input; they act as CO2 emitters on a global scale (Chen et al. 2013). On the other hand, coastal waters on the continental shelves are global CO2 sinks, where the influence of riverine inputs is reduced (Roobaert et al. 2019). Coastal waters can be both sources and sinks, depending on the influence of riverine inputs, land-use in their catchments, and the degree of in situ biological production (Chou et al. 2013; Kone et al. 2009; Kubo and Kanda 2020, St-Laurent et al. 2020).
The results of our seasonal survey show that Kanazawa Bay, which is a branched embayment of Tokyo Bay, acted as a net annual CO2 sink (Fig. 2e). The annual uptake of CO2 in Kanazawa Bay (estimated range: − 4.2 to − 2.1 mmol m−2 day−1) was lower than that in the entire Tokyo Bay (− 7.84 ± 7.40 mmol m−2 day−1; Tokoro et al. 2021). Annual average pCO2 in the surface layer (326 μatm) in this study was comparable to that in the entire bay (311 μatm), suggesting that the difference in wind conditions between the branched embayment and the main channel would create this difference in air–water CO2 fluxes. The coastal waters off the coast of Hannan absorbed CO2 during winter to spring (Table 1). The mean \({\text{F}}_{{{\text{CO}}_{{2}} }}\) during this period (estimated range: − 16.5 to − 5.3 mmol m−2 day−1) was similar to that in all of Osaka Bay (Endo et al. 2018). Tokoro et al. (2021) has shown that Osaka Bay acts as a net annual CO2 sink, like Tokyo Bay. Previous studies have proposed that efficient sewage treatment reduces the inorganic and organic carbon/nutrient ratio of incoming freshwater, which encourages net ecosystem production and the uptake of CO2 in urbanized coastal waters in Japan (Kubo and Kanda 2020; Kuwae et al. 2016). Because the catchment area of Japanese rivers is smaller than that of continental rivers, freshwater inflows are smaller relative to the volume of bays. This geological characteristic would form marine-dominated estuaries and contribute to the bays being net CO2 sinks (Kubo et al. 2017).
Conclusion
Our analyses distinguished and quantified the contribution of macrophytes from other factors (i.e., phytoplankton and freshwater–seawater mixing) to pCO2 and DOC dynamics in coastal waters. In this study, we showed that macrophyte habitats (i.e., macroalgae farms and seagrass beds) substantially affected in situ atmospheric CO2 removal and DOC supply during their growth periods. This finding implies that artificial macrophyte habitat creation (i.e., macroalgae farming and habitat restoration) has potential for direct atmospheric CO2 removal. Furthermore, we demonstrate that human-impacted coastal waters act as a net annual CO2 sink. In contrast, it is largely unquantified how much the spatial and temporal footprint of macrophyte metabolism substantially contributes to the annual-scale \({\text{F}}_{{{\text{CO}}_{{2}} }}\) and carbon sequestration. To understand the spatiotemporal footprint, future studies should incorporate biological metabolism, carbon sequestration processes, lateral and vertical advection, and timescales of atmosphere/ocean interactions (Bach et al. 2021; Hurd et al. 2022).
Data availability
All data generated or analyzed during this study are included in this article and its supporting Information file.
References
Bach LT, Tamsitt V, Gower J, Hurd CL, Raven JA, Boyd PW (2021) Testing the climate intervention potential of ocean afforestation using the great Atlantic Sargassum belt. Nat Commun 12:2556. https://doi.org/10.1038/s41467-021-22837-2
Bach LT, Ho DT, Boyd PW, Tyka MD (2023) Toward a consensus framework to evaluate air–sea CO2 equilibration for marine CO2 removal. Limnol Oceanogr Lett. https://doi.org/10.1002/lol2.10330
Baines SB, Pace ML (1991) The production of dissolved organic matter by phytoplankton and its importance to bacteria: patterns across marine and freshwater systems. Limnol Oceanogr 36:1078–1090. https://doi.org/10.4319/lo.1991.36.6.1078
Bakker P, Schmittner A, Lenaerts JTM, Abe-Ouchi A, Bi D, van den Broeke MR, Chan WL, Hu A, Beadling RL, Marsland SJ, Mernild SH, Saenko OA, Swingedouw D, Sullivan A, Yin J (2016) Fate of the Atlantic meridional overturning circulation: strong decline under continued warming and Greenland melting. Geophys Res Lett 43:12252–12260. https://doi.org/10.1002/2016GL070457
Barrón C, Duarte CM (2009) Dissolved organic matter release in a Posidonia oceanica meadow. Mar Ecol Prog Ser 374:75–84. https://doi.org/10.3354/meps07715
Bauer JE, Cai WJ, Raymond PA, Bianchi TS, Hopkinson CS, Regnier PAG (2013) The changing carbon cycle of the coastal ocean. Nature 504:61–70. https://doi.org/10.1038/nature12857
Buck-Wiese H, Andskog MA, Nguyen NP, Hehemann JH (2022) Fucoid brown algae inject fucoidan carbon into the ocean. Proc Natl Acad Sci USA 120:e2210561119. https://doi.org/10.1073/pnas.2210561119
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach. Springer, New York
Cai WJ (2011) Estuarine and coastal ocean carbon paradox: CO2 sinks or sites of terrestrial carbon incineration? Ann Rev Mar Sci 3:123–145. https://doi.org/10.1146/annurev-marine-120709-142723
Chen CTA, Huang TH, Chen YC, Bai Y, He X, Kang Y (2013) Air–sea exchanges of CO2 in the world’s coastal seas. Biogeosciences 10:6509–6544. https://doi.org/10.5194/bg-10-6509-2013
Chou WC, Gong GC, Cai WJ, Tseng CM (2013) Seasonality of CO2 in coastal oceans altered by increasing anthropogenic nutrient delivery from large rivers: evidence from the Changjiang-East China Sea system. Biogeosciences 10:3889–3899. https://doi.org/10.5194/bg-10-3889-2013
Cotovicz LC Jr, Knoppers BA, Brandini N, Costa Santos SJ, Abril G (2015) A strong CO2 sink enhanced by eutrophication in a tropical coastal embayment (Guanabara Bay, Rio de Janeiro, Brazil). Biogeosciences 12:6125–6146. https://doi.org/10.5194/bg-12-6125-2015
Cotovicz LC Jr, Knoppers BA, Régis CR, Tremmel D, Costa-Santos S, Abril G (2021) Eutrophication overcoming carbonate precipitation in a tropical hypersaline coastal lagoon acting as a CO2 sink (Araruama Lagoon, SE Brazil). Biogeochemistry 156:231–254. https://doi.org/10.1007/s10533-021-00842-3
Dafforn KA, Glasby TM, Airoldi L, Rivero NK, Mayer-Pinto M, Johnston EL (2015) Marine urbanization: an ecological framework for designing multifunctional artificial structures. Front Ecol Environ 13:82–90. https://doi.org/10.1890/140050
Dickson AG, Millero FJ (1987) A comparison of the equilibrium constants for the dissociation of carbonic acid in seawater media. Deep Sea Res A 34:1733–1743. https://doi.org/10.1016/0198-0149(87)90021-5
Duarte CM, Krause-Jensen D (2017) Export from seagrass meadows contributes to marine carbon sequestration. Front Mar Sci 4:13. https://doi.org/10.3389/fmars.2017.00013
Duarte CM, Bruhn A, Krause-Jensen D (2022) A seaweed aquaculture imperative to meet global sustainability targets. Nat Sustain 5:185–193. https://doi.org/10.1038/s41893-021-00773-9
Endo T, Shimano J, Ikenaga K, Kokubu H (2018) Estimation of air-sea CO2 fluxes in Osaka Bay, Harima-nada and Ago Bay on spatial distribution survey of dissolved inorganic carbon. J Jpn Soc Civ Eng. https://doi.org/10.2208/kaigan.74
Friedlingstein P, Jones MW, O’Sullivan M et al (2019) Global carbon budget 2019. Earth Syst Sci Data 11:1783–1838. https://doi.org/10.5194/essd-11-1783-2019
Froehlich HE, Afflerbach JC, Frazier M, Halpern BS (2019) Blue growth potential to mitigate climate change through seaweed offsetting. Curr Biol 29:3087–3093. https://doi.org/10.1016/j.cub.2019.07.041
Frontier N, de Bettignies F, Foggo A, Davoult D (2021) Sustained productivity and respiration of degrading kelp detritus in the shallow benthos: detached or broken, but not dead. Mar Environ Res 166:105277. https://doi.org/10.1016/j.marenvres.2021.105277
Griscom BW, Adams J, Ellis PW, Houghton RA, Lomax G, Miteva DA, Schlesinger WH, Shoch D, Siikamäki JV, Smith P, Woodbury P, Zganjar C, Blackman A, Campari J, Conant RT, Delgado C, Elias P, Gopalakrishna T, Hamsik MR, Herrero M, Kiesecker J, Landis E, Laestadius L, Leavitt SM, Minnemeyer S, Polasky S, Potapov P, Putz FE, Sanderman J, Silvius M, Wollenberg E, Fargione J (2017) Natural climate solutions. Proc Natl Acad Sci USA 114:11645–11650. https://doi.org/10.1073/pnas.1710465114
Han TT, Shi RJ, Qi ZH, Huang HH, Liang QY, Liu HX (2017) Interactive effects of oyster and seaweed on seawater dissolved inorganic carbon systems: implications for integrated multi-trophic aquaculture. Aquacult Environ Interact 9:469–478. https://doi.org/10.3354/aei00246
Hurd CL, Law CS, Bach LT, Britton D, Hovenden M, Paine ER, Raven JA, Tamsitt V, Boyd PW (2022) Forensic carbon accounting: assessing the role of seaweeds for carbon sequestration. J Phycol 58:347–363. https://doi.org/10.1111/jpy.13249
Ikawa H, Oechel WC (2015) Temporal variations in air-sea CO2 exchange near large kelp beds near SanDiego, California. J Geophys Res Ocean 120:50–63. https://doi.org/10.1002/2014JC010229
Inagaki S, Tanaka M, Akiyama S, Sakurai N, Rin B (1997) Field observations on flow and density structure in the Kanazawa-Hakkei area. Proc Coast Eng JSCE 44:376–380. https://doi.org/10.2208/proce1989.44.376
Jiang LQ, Cai WJ, Wang Y (2008) A comparative study of carbon dioxide degassing in river- and marine-dominated estuaries. Limnol Oceanogr 53:2603–2615. https://doi.org/10.4319/lo.2008.53.6.2603
Jiang Z, Fang J, Mao Y, Han T, Wang G (2013) Influence of seaweed aquaculture on marine inorganic carbon dynamics and sea-air CO2 flux. J World Aquacul Soc 44:133–140. https://doi.org/10.1111/jwas.12000
Kondo J (2000) Atmosphere science near the ground surface. University of Tokyo Press, Tokyo
Koné YJM, Abril G, Kouadio KN, Delille B, Borges AV (2009) Seasonal variability of carbon dioxide in the rivers and lagoons of ivory coast (West Africa). Estuaries Coasts 32:246–260. https://doi.org/10.1007/s12237-008-9121-0
Koweek DA, Nickols KJ, Leary PR, Litvin SY, Bell TW, Luthin T, Lummis S, Mucciarone DA, Dunbar RB (2017) A year in the life of a central California kelp forest: physical and biological insights into biogeochemical variability. Biogeosciences 14:31–44. https://doi.org/10.5194/bg-14-31-2017
Krause-Jensen D, Duarte CM (2016) Substantial role of macroalgae in marine carbon sequestration. Nat Geosci 9:737–742. https://doi.org/10.1038/NGEO2790
Kubo A, Kanda J (2020) Coastal urbanization alters carbon cycling in Tokyo Bay. Sci Rep 10:20413. https://doi.org/10.1038/s41598-020-77385-4
Kubo A, Yamamoto-Kawai M, Kanda J (2015) Seasonal variations in concentration and lability of dissolved organic carbon in Tokyo Bay. Biogeosciences 12:269–279. https://doi.org/10.5194/bg-12-269-2015
Kubo A, Maeda Y, Kanda JA (2017) Significant net sink for CO2 in Tokyo Bay. Sci Rep 7:44355. https://doi.org/10.1038/srep44355
Kuwae T, Crooks S (2021) Linking climate change mitigation and adaptation through coastal green–gray infrastructure: a perspective. Coast Eng J 63:188–199. https://doi.org/10.1080/21664250.2021.1935581
Kuwae T, Kanda J, Kubo A, Nakajima F, Ogawa H, Sohma A, Suzumura M (2016) Blue carbon in human-dominated estuarine and shallow coastal systems. Ambio 45:290–301. https://doi.org/10.1007/s13280-015-0725-x
Kuwae T, Kanda J, Kubo A, Nakajima F, Ogawa H, Sohma A, Suzumura M (2019) CO2 uptake in the shallow coastal ecosystems affected by anthropogenic impacts. In: Kuwae T, Hori M (eds) Blue carbon in shallow coastal ecosystems. Springer, Singapore, pp 295–329
Kuwae T, Watanabe A, Yoshihara S, Suehiro F, Sugimura Y (2022) Implementation of blue carbon offset crediting for seagrass meadows, macroalgal beds, and macroalgae farming in Japan. Mar Pol 138:104996. https://doi.org/10.1016/j.marpol.2022.104996
Kuwagata T, Kondo J (1990) Estimation of aerodynamics roughness at the regional meteorological stations (AMeDAS) in the central part of Japan. TENKI 37:197–201
Laruelle GG, Dürr HH, Slomp CP, Borges AV (2010) Evaluation of sinks and sources of CO2 in the global coastal ocean using a spatially-explicit typology of estuaries and continental shelves. Geophys Res Lett 37:L15607. https://doi.org/10.1029/2010GL043691
Laruelle GG, Goossens N, Arndt S, Cai W-J, Regnier P (2017) Air–water CO2 evasion from US east coast estuaries. Biogeosciences 14:2441–2468. https://doi.org/10.5194/bg-14-2441-2017
Lauerwald R, Laruelle GG, Hartmann J, Ciais P, Regnier PAG (2015) Spatial patterns in CO2 evasion from the global river network. Glob Biogeochem Cycles 29:534–554. https://doi.org/10.1002/2014GB004941
Lewis E, Wallace D, Allison LJ (1998) Program developed for CO2 system calculations. United States. https://doi.org/10.2172/639712
Li H, Zhang Z, Xiong T, Tang K, He C, Shi Q, Jiao N, Zhang Y (2022) Carbon sequestration in the form of recalcitrant dissolved organic carbon in a seaweed (kelp) farming environment. Environ Sci Technol 56:9112–9122. https://doi.org/10.1021/acs.est.2c01535
Lin WJ, Chiu MC, Lin CW, Lin HJ (2021) Effects of sediment characteristics on carbon dioxide fluxes based on interacting factors in unvegetated tidal flats. Front Mar Sci 8:670180. https://doi.org/10.3389/fmars.2021.6701
Macreadie PI, Anton A, Raven JA, Beaumont N, Connolly RM, Friess DA, Kelleway JJ, Kennedy H, Kuwae T, Lavery PS, Lovelock CE, Smale DA, Apostolaki ET, Atwood TB, Baldock J, Bianchi TS, Chmura GL, Eyre BD, Fourqurean JW, Hall-Spencer JM, Huxham M, Hendriks IE, Krause-Jensen D, Laffoley D, Luisetti T, Marbà N, Masque P, McGlathery KJ, Megonigal JP, Murdiyarso D, Russell BD, Santos R, Serrano O, Silliman BR, Watanabe K, Duarte CM (2019) The future of blue carbon science. Nat Commun 10:3998. https://doi.org/10.1038/s41467-019-11693-w
Maher DT, Eyre BD (2012) Carbon budgets for three autotrophic Australian estuaries: implications for global estimates of the coastal air-water CO2 flux. Glob Biogeochem Cycles. https://doi.org/10.1029/2011GB004075
McGillis WR, Edson JB, Hare JE, Fairall CW (2001) Direct covariance air-sea CO2 fluxes. J Geophys Res Oceans 106:16729–16745. https://doi.org/10.1029/2000JC000506
McLeod E, Chmura GL, Bouillon S, Salm R, Björk M, Duarte CM, Lovelock CE, Schlesinger WH, Silliman BR (2011) A blueprint for blue carbon: toward an improved understanding of the role of vegetated coastal habitats in sequestering CO2. Front Ecol Environ 9:552–560. https://doi.org/10.1890/110004
Ministry of the Environment (1996) National survey on the Natural Environment 5th survey. Ministry of the Environment, Tokyo
Murray K, Conner MM (2009) Methods to quantify variable importance: implications for the analysis of noisy ecological data. Ecology 90:348–355. https://doi.org/10.1890/07-1929.1
Otani S, Endo T (2019) CO2 flux in tidal flats and salt marshes. In: Kuwae T, Hori M (eds) Blue carbon in shallow coastal ecosystems. Springer, Singapore, pp 223–250
Pessarrodona A, Assis J, Filbee-Dexter K, Burrows MT, Gattuso JP, Duarte CM, Krause-Jensen D, Moore PJ, Smale DA, Wernberg T (2022) Global seaweed productivity. Sci Adv. https://doi.org/10.1126/sciadv.abn2465
Pessarrodona A, Franco-Santos RM, Wright LS, Vanderklift MA, Howard J, Pidgeon E, Wernberg T, Filbee-Dexter K (2023) Carbon sequestration and climate change mitigation using macroalgae: a state of knowledge review. Biol Rev 98:1945–1971. https://doi.org/10.1111/brv.12990
R Core Team (2022) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Raymond PA, Cole JJ (2001) Gas exchange in rivers and estuaries: choosing a gas transfer velocity. Estuaries 24:312–317. https://doi.org/10.2307/1352954
Regnier P, Friedlingstein P, Ciais P, Mackenzie FT, Gruber N, Janssens IA, Laruelle GG, Lauerwald R, Luyssaert S, Andersson AJ, Arndt S, Arnosti C, Borges AV, Dale AW, Gallego-Sala A, Goddéris Y, Goossens N, Hartmann J, Heinze C, Ilyina T, Joos F, LaRowe DE, Leifeld J, Meysman FJR, Munhoven G, Raymond PA, Spahni R, Suntharalingam P, Thullner M (2013) Anthropogenic perturbation of the carbon fluxes from land to ocean. Nat Geosci 6:597–607. https://doi.org/10.1038/ngeo1830
Roobaert A, Laruelle GG, Landschützer P, Gruber N, Chou L, Regnier P (2019) The spatiotemporal dynamics of the sources and sinks of CO2 in the global coastal ocean. Glob Biogeochem Cycles 33:1693–1714. https://doi.org/10.1029/2019GB006239
Ross FWR, Boyd PW, Filbee-Dexter K, Watanabe K, Ortega A, Krause-Jensen D, Lovelock C, Sondak CFA, Bach LT, Duarte CM, Serrano O, Beardall J, Tarbuck P, Macreadie PI (2023) Potential role of seaweeds in climate change mitigation. Sci Total Environ 885:163699. https://doi.org/10.1016/j.scitotenv.2023.163699
St-Laurent P, Friedrichs MAM, Najjar RG, Shadwick EH, Tian H, Yao Y (2020) Relative impacts of global changes and regional watershed changes on the inorganic carbon balance of the Chesapeake Bay. Biogeosciences 17:3779–3796. https://doi.org/10.5194/bg-17-3779-2020
Takahashi T, Sutherland SC, Wanninkhof R, Sweeney C, Feely RA, Chipman DW, Hales B, Friederich G, Chavez F, Sabine C, Watson A, Bakker DCE, Schuster U, Metzl N, Yoshikawa-Inoue H, Ishii M, Midorikawa T, Nojiri Y, Körtzinger A, Steinhoff T, Hoppema M, Olafsson J, Arnarson TS, Tilbrook B, Johannessen T, Olsen A, Bellerby R, Wong CS, Delille B, Bates NR, de Baar HJW (2009) Climatological mean and decadal change in surface ocean pCO2, and net sea-air CO2 flux over the global oceans. Deep Sea Res 56:554–577. https://doi.org/10.1016/j.dsr2.2008.12.009
Terada R, Abe M, Abe T, Aoki M, Dazai A, Endo H, Kamiya M, Kawai H, Kurashima A, Motomura T, Murase N, Sakanishi Y, Shimabukuro H, Tanaka J, Yoshida G, Aoki M (2021) Japan’s nationwide long-term monitoring survey of seaweed communities known as the “Monitoring Sites 1000”: Ten-year overview and future perspectives. Phycol Res 69:12–30. https://doi.org/10.1111/pre.12395
Tokoro T, Kuwae T (2022) Air-water CO2 and water-sediment O2 exchanges over a tidal flat in Tokyo Bay. Front Mar Sci 9:989270. https://doi.org/10.3389/fmars.2022.989270
Tokoro T, Hosokawa S, Miyoshi E, Tada K, Watanabe K, Montani S, Kayanne H, Kuwae T (2014) Net uptake of atmospheric CO2 by coastal submerged aquatic vegetation. Glob Change Biol 20:1873–1884. https://doi.org/10.1111/gcb.12543
Tokoro T, Watanabe K, Tada K, Kuwae T (2019) Air-–Water CO2 flux in shallow coastal waters: theory, methods, and empirical studies. In: Kuwae T, Hori M (eds) Blue carbon in shallow coastal ecosystems. Springer, Singapore, pp 153–184
Tokoro T, Nakaoka S, Takao S, Kuwae T, Kubo A, Endo T, Nojiri Y (2021) Contribution of biological effects to carbonate-system variations and the air–water CO2 flux in urbanized bays in Japan. J Geophys Res Oceans. https://doi.org/10.1029/2020JC016974
Wada S, Aoki MN, Tsuchiya Y, Sato T, Shinagawa H, Hama T (2007) Quantitative and qualitative analyses of dissolved organic matter released from Ecklonia cava Kjellman, in Oura Bay, Shimoda, Izu Peninsula, Japan. J Exp Mar Biol Ecol 349:344–358. https://doi.org/10.1016/j.jembe.2007.05.024
Wanninkhof R (2014) Relationship between wind speed and gas exchange over the ocean revisited. Limnol Oceanogr Methods 12:351–362. https://doi.org/10.4319/lom.2014.12.351
Watanabe K, Kuwae T (2015) How organic carbon derived from multiple sources contributes to carbon sequestration processes in a shallow coastal system? Glob Change Biol 21:2612–2623. https://doi.org/10.1111/gcb.12924
Watanabe K, Yoshida G, Hori M, Umezawa Y, Moki H, Kuwae T (2020) Macroalgal metabolism and lateral carbon flows can create significant carbon sinks. Biogeosciences 17:2425–2440. https://doi.org/10.5194/bg-17-2425-2020
Weigel BL, Pfister CA (2021) The dynamics and stoichiometry of dissolved organic carbon release by kelp. Ecology 102:e03221. https://doi.org/10.1002/ecy.3221
Weiss RF (1974) Carbon dioxide in water and seawater: the solubility of a non-ideal gas. Mar Chem 2:203–215. https://doi.org/10.1016/0304-4203(74)90015-2
Wu J, Zhang H, Pan Y, Krause-Jensen D, He Z, Fan W, Xiao X, Chung I, Marbà N, Serrano O, Rivkin RB, Zheng Y, Gu J, Zhang X, Zhang Z, Zhao P, Qiu W, Chen G, Duarte CM (2020) Opportunities for blue carbon strategies in China. Ocean Coast Manag 194:105241. https://doi.org/10.1016/j.ocecoaman.2020.105241
Yao H, McCutcheon MR, Staryk CJ, Hu X (2020) Hydrologic controls on CO2 chemistry and flux in subtropical lagoonal estuaries of the northwestern Gulf of Mexico. Limnol Oceanogr 65:1380–1398. https://doi.org/10.1002/lno.11394
Yates KK, Dufore C, Smiley N, Jackson C, Halley RB (2007) Diurnal variation of oxygen and carbonate system parameters in Tampa Bay and Florida Bay. Mar Chem 104:110–124. https://doi.org/10.1016/j.marchem.2006.12.008
Yoneda Y, Yoshida T, Shiba S, Matsui K, Kaneko K, Suzuki T, Takebe T (2014) Fluctuation in standing stock of seaweed beds formed on a sloped seawall reef and its associating environmental factors in Osaka Bay. J Fish Eng 50:151–162. https://doi.org/10.18903/fisheng.50.3_151
Zeebe RE, Wolf-Gladrow D (2001) CO2 in Seawater: Equilibrium, Kinetics, Isotopes. Elsevier, Amsterdam
Acknowledgements
We acknowledge the support in field surveys by the Yokohama city Blue Carbon Project funded by Carbon Neutral Cities Alliance. We thank H. Kimishima, R. Makino, and N. Umegaki from Port and Airport Research Institute for chemical analyses. Finally, we thank two anonymous reviewers for their useful comments that contributed to the development of the paper.
Funding
This study was funded in part by Grants-in-Aid for Scientific Research (KAKENHI) Grant Numbers 19K20500, 22H03798, and 22H05738 from the Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Contributions
KW and TK contributed to the study conception and design. Field surveys were conducted by KW, TT, and HM. Sample processing and analysis were performed by KW. The first draft of the manuscript was written by KW and all authors commented on the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no competing interest to declare that are relevant to the content of this article.
Additional information
Responsible Editor :Klaus-Holger Knorr.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Watanabe, K., Tokoro, T., Moki, H. et al. Contribution of marine macrophytes to pCO2 and DOC variations in human-impacted coastal waters. Biogeochemistry 167, 831–848 (2024). https://doi.org/10.1007/s10533-024-01140-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-024-01140-4