Abstract
Non-native ungulates (sheep, goats, and pigs) have significant negative impacts on ecosystem biodiversity, structure, and biogeochemical function throughout the Pacific Islands. Elevated nitrogen (N) availability associated with ungulate disturbance has been shown to promote the success of resource-exploitive invasive plants. While ungulate removal is a common restoration intervention, evaluations of its efficacy typically focus on vegetation responses, rather than underlying nutrient cycling. We used multiple chronosequences of ungulate exclusion (10–24 years duration) in three Hawaiian ecosystems (montane wet forest, dry forest, and dry shrubland) to determine N cycle recovery by characterizing gross mineralization and nitrification, soil inorganic N concentrations and leaching, N2O emissions, and plant tissue δ15N. Ungulate removal led to a 1–2 ‰ decline in foliar δ15N in most species, consistent with a long-term decrease in N fractionation via ecosystem N losses, or a shift in the relative turnover of N forms. This interpretation was supported by significant (dry forest) or trending (wet forest) increases in mineralization and decreases in nitrification, but conflicts with lack of observed change in inorganic N pool sizes or gaseous losses, and increased leaching in the dry forest. While results could indicate that ungulate invasions do not strongly impact N cycling in the first place (no uninvaded control sites exist in Hawai’i to test this hypothesis), this would be inconsistent with observations from other sites globally. Instead, impacts may be spatially patchy across the landscape, or ungulate invasions (possibly in combination with other disturbances) may have permanently shifted biogeochemical function or decoupled elemental cycles. We conclude that eliminating ungulate disturbance alone may not achieve restoration goals related to N cycling within the timeframe examined here.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The extent and timing of biogeochemical change following anthropogenic disturbance is increasingly recognized as an essential consideration for designing interventions for ecosystem recovery (McLauchlan et al. 2007; Sullivan et al. 2019; Wong et al. 2019). In many ecosystems, non-native ungulates cause extensive disturbance and disrupt structure, biodiversity, and biogeochemical function (Mack and D’Antonio 1998; Campbell and Long 2009; Spear and Chown 2009; Nogueira-Filho et al. 2009; Wardle and Peltzer 2017). There is evidence that ungulate impacts on soil structure and vegetation composition can lead to substantially increased rates of biogeochemical cycling, especially of nitrogen (N; Siemann et al. 2009; Long et al. 2017), with the bulk of such observations coming from temperate ecosystems (Cushman et al. 2004; Cuevas et al. 2012; Wirthner et al. 2012; Bueno et al. 2013). Ungulate impacts may be particularly significant for ecosystems that are characterized by generally low pre-invasion nutrient availability, and where elevated N availability may promote further ecosystem degradation by favoring the success of resource-intensive non-native plant species that outcompete resource-conservative native plant communities (Ostertag and Verville 2002; Daehler 2003; Funk 2008; Knauf et al. 2021). Attempts to restore ecosystem function via ungulate removal must, therefore, critically assess the degree of recovery of biogeochemical functioning.
Across the Hawaiian archipelago, non-native ungulate disturbance is widespread; wetter ecosystems are heavily colonized by feral pigs, and drier ecosystems by pigs as well as feral sheep and goats (Chynoweth et al. 2013; Wehr et al. 2018). Though non-native ungulates are destructive across a wide variety of ecosystems globally (Wardle and Peltzer 2017), effects may be especially pronounced because these remote islands evolved in the absence of any native ungulates (Hess 2016). Changes to vegetation and soil physical and chemical properties with invasion have probable– though not well-characterized– implications for the cycling, storage, and stoichiometry of carbon (C) and nutrients. Feral pigs have been shown to reduce vegetation biomass and regeneration (through herbivory and trampling), fragment litter and mix soils during rooting and foraging activities, compact soil and reduce infiltration, create intensely disturbed patches such as wallows, and create nutrient-enriched patches through urine and feces (Barrios-Garcia and Ballari 2012; Murphy et al. 2013; Long et al. 2017; Fortini et al. 2020). In areas of Hawaiian wet forest with greater evidence of pig activity (i.e., digs and wallows), soils were wetter and had greater bulk density, a lower C:N ratio, lower labile C, and higher soil pH, as well as a greater concentration of ammonium (NH4+) than less-affected areas (Long et al. 2017). Feral sheep and goats defoliate and trample vegetation, as well as increase soil erosion as a result of compaction, reduced vegetation cover, and reduced water infiltration (Chynoweth et al. 2013; Ortíz-Alcaraz et al. 2016; Hess et al. 2017; Gizicki et al. 2017). Reduced vegetation cover may lower vegetative nutrient demand (sink strength) and inputs of organic matter as litter, with inputs that do occur decomposing more quickly as a result of increased fragmentation (Singer et al. 1984). Compaction and changes to soil moisture may also affect soil redox conditions, with expected effects on microbial-mediated nutrient cycling processes (Wehr et al. 2019). In Hawaiʻi, pig activity is associated with reduced bacterial microbial diversity (Wehr et al. 2019) and changes in the abundance and diversity of soil macroinvertebrates such as invasive earthworms. In addition to direct effects, ungulates may have synergistic impacts with invasive non-native plant species; ungulates create microsite disturbances that facilitate invasion, and also act as propagule vectors (Nogueira-Filho et al. 2009; Cole and Litton 2013). As well as benefiting from increased nutrient availability to facilitate establishment, non-native plant species may further impact biogeochemistry through their distinct (often more nutrient-rich) tissue stoichiometry and faster decomposition rates (Mack and D’Antonio 2003; Allison and Vitousek 2004; Penuelas et al. 2009). At the broader scale, disturbances that result in elevated ecosystem N availability may drive increased ‘leakiness’, accelerating ecosystem N losses to freshwaters and as greenhouse gases such as nitrous oxide (N2O; Vitousek et al. 1979).
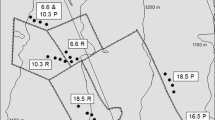
Removal and exclusion of ungulates via fencing is a common management practice aimed at conserving native species and restoring ecosystem function (Peh et al. 2015; Ortíz-Alcaraz et al. 2016; Gizicki et al. 2017; Hata et al. 2014). On the Island of Hawaiʻi, exclusion structures have been maintained for up to three decades (Cabin et al. 2000; Nogueira-Filho et al. 2009; Hess et al. 2017). While this intervention is labor and cost intensive, the short-term benefits (such as positive impacts on abundance and growth of native species) are well established (e.g., Scowcroft and Giffin 1983; Scowcroft and Sakai 1983; Cabin et al. 2000), and viewed as essential for successful restoration (Fig. 1). However, studies on the magnitude and timing of responses have largely focused on vegetation (e.g., Cole et al. 2012; Cole and Litton 2013), with few examining underlying biogeochemical responses, and long-term outcomes are not well quantified (Cabin et al. 2000). This knowledge gap is notable as soil functioning is likely to strongly impact the trajectory of vegetation recovery. Available evidence suggests that exclusion of pigs from Hawaiian wet forests can decrease bare soil and erosion (Bruland et al. 2010; Cole and Litton 2013), and in one study (Long et al. 2017) tended to shift the system toward increased abundance of nitrate (NO3−), a highly mobile form of N that is more readily leached from the soil profile. Increasing time since ungulate exclusion (from 6 to 18 years) was associated with a decrease in soil N mineralization and pH and increase in nitrification. When all exclusion plots of varying ages were contrasted with those still frequented by pigs, exclusion decreased bulk density and volumetric water content and increased base cations, but did not otherwise affect total, labile or organic N and C (Long et al. 2017).
Ungulate impacts are frequently accompanied by a multitude of other disturbances such as increased habitat fragmentation, non-native plant invasions, and invasions by other animals such as rodents (Cabin et al. 2000). Interactions between ungulate removal and disturbances may maintain disruption to system biogeochemistry, or even shift ecosystems into new or alternative steady states, at least on longer (decadal-centennial) time scales. For example, feral pig exclusion in Hawaiian wet forest resulted in increased biomass of non-native plants such as Kahili ginger (Hedychium gardnerianum) and strawberry guava (Psidium cattleyanum; Cole et al. 2012; Cole and Litton 2013). In dry forests, ungulate exclusion increases native plant abundance and diversity, but continuing dominance of highly invasive non-native grasses suppresses woody recruitment (Cabin et al. 2000). In Hawaiian dry systems, harvesting of native forest trees, the introduction of fire prone non-native invasive grasses, and expanding populations of feral goats and sheep have all conspired to create a novel fire regime that maintains non-native grass-wildfire dominated savanna (Ellsworth et al. 2014). Impacts of feral ungulates on both plant communities and biogeochemical processes may thus feedback on each other to maintain invaded systems in an altered ecological state that persists for at least some time following removal.
Taken together, the diversity of complex interactions may explain the inconsistent responses to non-native ungulate removal observed across ecosystems. For example, Cushman et al. (2004) and Tierney and Cushman (2006) documented no changes in net mineralization, nitrification or inorganic N pools after four to five years of pig exclusion in California grasslands, while Siemann et al. (2009) observed that pig exclusion increased soil C:N ratios in a Texas woodland, possibly reflecting a decrease in litter incorporation and mineralization, while others have observed elevated nitrate in temperate forests (Singer et al. 1984). Disturbances such as soil loss may continue even long after ungulate eradication due to persistent changes in vegetation cover (Gizicki et al. 2017). These variable trajectories across sites may reflect diverse combinations of disturbance type, severity, duration, and underlying differences such as initial nutrient availability, with the strength of biogeochemical impacts also expected to differ by ecosystem type. Specifically, wetter (e.g., montane tropical rainforest) versus drier (e.g., dry tropical woodland or shrubland) ecosystems differ in both the dominant ungulate species present and in their vegetation/soil types, structure, and resource availability. Comparing diverse ecosystems is important because, for example, in drier woodland and shrubland ecosystems water may be a stronger control than nutrients on plant productivity and diversity (Cole et al. 2021).
In this study, we asked whether long-term ungulate exclusion alters biogeochemical cycling across three Hawaiian ecosystems. Studies comparing more- to less-heavily disturbed areas (Long et al. 2017) provide reasonably compelling evidence that ungulate disturbance changes biogeochemical cycling. However, because there are no true unimpacted ‘control’ areas in Hawaiʻi, we cannot answer directly questions about pre-invasion nutrient cycling, including whether pre-invasion cycling can be ‘restored’ through ungulate exclusion. Instead, we tested the hypothesis that removal of non-native ungulates reduces rates of N cycling and ecosystem N loss, thereby creating a more closed N cycle with lower N availability following ungulate removal, with increased plant biomass simultaneously driving increased plant N demand. We sampled 11 fenced exclosures where ungulates had been excluded from Metrosideros-dominated tropical montane wet forest from 1 to 24 years, Metrosideros-dominated tropical dry forest (exclusion from 1 to 12 years) and Dodonaea-dominated tropical shrubland (10 years), along with paired unfenced controls. We measured a range of short- and long-term soil N cycling and availability metrics, including resin-available inorganic N, inorganic N leaching, gross mineralization and nitrification rates, soil N2O fluxes, and vegetation N isotopic ratios (as integrated measures of ecosystem N losses) to determine whether and over what time scales ungulate exclusion impacts soil N cycling and availability.
Materials and methods
Study site
Field sampling was conducted in 2016/2017 across three widespread vegetation types on the Island of Hawaiʻi, HI (the largest island in the Hawaiian archipelago), where ungulate removal treatments had been in place for variable durations. We established adjacent control areas where feral ungulates continued to be present (Fig. S1). Sites representing two locally common vegetation types– Metrosideros dry woodland and Dodonaea shrubland– were located in the Pōhakuloa Training Area (19°40′ N, 155°20′ W). Tropical montane wet forest plots were located in an chronosequence of feral pig removal units (and adjacent sites with pigs present) in Hawaiʻi Volcanoes National Park and the adjacent Pu’u Maka’ala Natural Area Reserve (19°24′ N, 155°8′ W; Cole and Litton 2013; Fig. 1).
The shrubland and dry forest site sites are situated at 1500 m a.s.l. with a mean annual temperature of 13 °C. Total annual precipitation in 2016 was 414 mm for the shrubland (5 year average 310 mm) and 400 mm for the dry forest (5 year average 420 mm). Substrate is classified as isothermic Humic Haplustands; deep, well drained soils that formed in basic volcanic ash in `a`a lava. The Dodonaea viscoa open shrubland is largely composed of native shrubs, D. viscosa and Chenopodium oahuense, and native C4 grass, Eragrostis atropioides (Poaceae). The region is heavily invaded by nonnative C4 grass, Cenchrus setaceus (fountain grass) and invasive forb, Senecio madagascariensi (fireweed). Goats, and to a lesser extent sheep, are present at both sites. Two feral ungulate exclosures in the shrubland were established 1 year or 10 years prior to the start of sampling, with the former having fences installed but no active ungulate removal until immediately prior to the study. The former is used here as a proxy for ungulate presence. The dry forest site is a Metrosideros polymorpha woodland with scattered shrubs of D. viscoa and Styphelia tameiameiae and invasive forb, S. madagascariensi. In this dry forest ecosystem, sampling sites consisted of one ungulate-present control, plus exclosure plots of ages 1, 5.5, 8, and 12 years. All study sites were located in areas of comparable climate, vegetation, and substrate (Tables S1 and S2).
The wet forest sites were located ~ 1200 m a.s.l., with a mean annual temperature of 14–16 °C, and mean annual precipitation of 3985 mm with no distinct seasonality (Giambelluca et al. 2013). Substrate is classified as Lithic Hapludands; moderately well-drained soils formed in volcanic ash deposited over Pahoehoe lava. Forest cover in all sites is classified as M. polymorpha (overstory tree)/Cibotium spp. (mid-story tree fern) tropical montane wet forest (Wagner et al. 1999). Sampling was conducted in a paired design at six sites from which ungulates had been excluded for 1, 12, 14, 16, 22 or 24 years. Study sites were located in areas of similar climate, vegetation, and substrate (Table S1; the one exception was the 1-year-old exclosure, which had an understory invaded by the nonnative ginger species, Hedychium gardnerianum). Paired sets of four plots were established inside and outside of the ungulate exclosures (Table S1), so that each exclosure was matched with a nearby set of plots where ungulates were still present. Two of the exclosures (Lava and NLM) shared one paired set of control (ungulates present) plots (PMA, Table S1).
Within each control or exclosure site, four 18 m-radius circular sampling plots were established. Sample size values below refer to the number of samples taken within each of these sampling plot units.
Soil sampling
Soil N2O flux was measured in June 2017 using a static chamber method. Eight collars were placed in each of the four plots in all exclosure and control sites. Collars were 20 cm diameter in the wet forest and 10 cm diameter in the shrubland and dry forest because soils were shallow and rocky. Chambers were topped with 10 cm tall PVC lids with a septa sampling port and a vent (path length 60 cm) to allow pressure equalization. Headspace samples (20 ml) were drawn at 0, 30, and 60 min. Samples were taken at ambient soil moisture (all sites), and again 24 h after the addition of 2.5 cm rainfall equivalent in the dry forest and shrubland. Samples were analyzed at Cornell University using a gas chromatograph fitted with electron capture and thermal conductivity detectors (Shimadzu GC-2014, Shimadzu Corp. Kyoto, Japan). Four depth measurements inside of the collar were averaged to calculate chamber volume. Fluxes were calculated based on the linear rate of increase in concentration over time.
In June-July 2015, soils from dry forest and shrubland were sampled using 12 randomly-located, 3 cm diameter by 5 cm deep cores taken from with each plot and processed for bulk density. A wide suite of soil physical and chemical properties were assessed in the wet forest prior to the initiation of this study (Long et al. 2017).
Soil gross N mineralization and nitrification rates were quantified using the 15N pool dilution method (modified from Hart et al. 1994a, b) once per site between Feb and Apr 2016. Four 5 cm diameter cores (0–5 cm depth, without litter) per plot were collected, composited, and split into three treatments, receiving an aqueous solution of 99% enriched (15NH4)2SO4, K15NO3, or deionized water (negative control). Solution volumes were adjusted to limit changes to percent soil water content to 1–2%. Label quantity was adjusted based on previous measures of NH4+ and NO3− concentrations in study plots (Long et al. 2017) to ~ 15% of the starting concentration. Immediately after homogenizing labeled soils, two replicate subsamples from each treatment were extracted in 2:1 2 M KCl. Two additional subsamples were extracted after 12 h of incubation at 25 ºC. Inorganic N concentrations in extracts were quantified colorimetrically with a Lachat QuickChem (Hach Co., Saskatoon, Canada) at the University of Hawaiʻi Hilo Analytical Laboratory. A microdiffusion method was used to determine δ15N of NH4+ and NO3− as per Stephan and Kavanagh (2009). Samples were diffused for 7 d at 22 °C with sample volume adjusted so that sample + spike did not exceed 35 µg N (for optimal N recovery) and filters were analyzed for δ15N using a continuous flow isotope ratio mass spectrometer (Model Delta V Advantage; Thermo‐Scientific, Waltham, USA) coupled to an elemental analyzer (Carlo Erba Model NC2500) at the Cornell University Stable Isotope Laboratory. Control diffusions were run to account for reagent N contamination, and samples with incomplete recovery were excluded from analysis. Gross fluxes were calculated using equations as per Hart et al. (1994a, b).
Soil NO3− and NH4+ availability was estimated using the ion-exchange resin bag method (Binkley and Matson 1983). Because resins mimic nutrient uptake by plants, they provide a useful measure of nutrient supply, and resin-captured NO3− has been shown to be highly correlated with both soil NO3− concentrations and rates of net nitrification (Subler et al. 1995). Four nylon mesh bags per plot containing 10 g of mixed bed cation–anion resin (Sigma Aldrich, St. Louis, MO) were placed at the litter layer-mineral soil interface and incubated for either 3 months (wet forest) or 5 months (dry forest and shrubland). During the incubations, control bags were stored both in the field (in sealed plastic bags) and in the laboratory (refrigerator controls). Upon collection, bags were cleaned by thoroughly rinsing with distilled water, and extracted by shaking with 100 mL 2 M KCl for two hours. Extracts were analyzed for NO3− and NH4+ concentration as described above.
Resin-based soil solution lysimeters allow for the calculation of annual N leachate fluxes by isolating the resin from direct soil contact and quantifying the concentration of N in free percolating soil water. One lysimeter was installed in each wet forest plot at a depth of 30 cm. A pit was excavated and the lysimeter installed laterally into the soil profile. Resins were collected after three months and extracted and analyzed as described above. Due to concerns over unexploded military ordnance in the dry forest and shrubland sites, resin strips (PRS™-probes Western Ag, Saskatoon, Saskatchewan Canada) were installed by placing strips horizontally at a depth of 10 cm. This method allows for the calculation of a mass per area of leaching (kg ha−1). Strips were retrieved after three months, rinsed with DI water, and stored at 4 °C until analysis.
Foliar isotopic sampling
Because foliar δ15N integrates all microbial and plant transformations of soil N until it is laid down in leaves, foliar isotope ratios were used to explore broad changes in N cycling (Evans 2001). Foliar samples were collected from each plot in June 2017: composite samples were hand-collected from three individuals of three species at each site, with the exception of one wet forest site where only two species were consistently present. In the wet forest, species selected included the native tree fern Cibotium glaucum, and shrubs Coprosma ochracea and Broussaisia argute (understory species). In the dry forest, species were native shrubs D. viscosa and S. tameiameiae and the nonnative forb S. madagascariensi and in the shrubland, species included native shrubs C. oahuense, D. viscosa, and S. madagascariensis (all sun-leaves). Samples were oven-dried at 65 °C and ground using a ball mill and the isotopic composition determined as described above.
Statistical analyses
Statistical analyses were conducted in JMP Pro 13.1 (SAS Institute, USA). Mixed models were used to assess the effect of duration of ungulate exclusion on response variables. Duration of exclusion was included as a fixed effect, and site and plot (nested within site) as random effects. For paired sites in the wet forest (where each exclosure site was paired with a nearby unfenced control site) the pair, site nested within pair, and site nested within pair within plot were also included as random variables. Because of the paired design for the wet forest, data were also analyzed with ungulate presence or absence as a fixed effect (i.e., all exclusion sites combined, regardless of duration) and pair as a random effect. Data from these combined analyses are reported only where differences are significant. Model residuals were inspected, and where necessary, data were log transformed or Johnson Su transformed to meet assumptions of normality. Significance was set at alpha = 0.05.
Results
Soil N 2 O fluxes
There was no significant change in N2O fluxes with increasing time since ungulate exclusion in any of the three ecosystems (shrubland with ambient moisture; P < 0.09, shrubland with water addition; P < 0.56, dry forest with ambient moisture; P < 0.18, dry forest with water addition; P < 0.34, or wet forest; P < 0.17; Figs. 2a, b, 3a, b, and 4a).
Metrics of nitrogen availability and cycling in a Hawaiian Dodonea shrubland with time since ungulate exclusion. (a) soil N2O flux under ambient soil moisture, (b) soil N2O flux after a simulated rainfall event, (c) 15N dilution mineralization and nitrification rates, (d) resin total inorganic N (NH4+ and NO3−), (e) lysimeter total inorganic N (NH4+ and NO3−) fluxes and (f) foliar N isotope ratio (δ15N). Colored lines indicate significant linear relationships between variables
Metrics of nitrogen availability and cycling in a Hawaiian Metrosideros dry woodland with time since ungulate exclusion. (a) soil N2O flux under ambient soil moisture, (b) soil N2O flux after a simulated rainfall event, (c) 15N dilution-measured mineralization and nitrification rates, (d) resin total inorganic N (NH4+ and NO3−), (e) total lysimeter inorganic N (NH4+ and NO3−) fluxes and (f) foliar N isotope ratios (δ15N). Colored lines indicate significant relationships between variables
Metrics of nitrogen availability and cycling in a Hawaiian wet tropical montane forest with time since ungulate exclusion. (a) soil N2O flux under ambient soil moisture, (b) soil N2O flux after a simulated rainfall event, (c) 15N dilution-measured mineralization and nitrification rates, (d) resin total inorganic N (NH4+ and NO3−), (e) total lysimeter inorganic N (NH4+ and NO3−) fluxes and (f) foliar N isotope ratios (δ15N). Colored lines indicate significant linear relationships between variables
Mineralization and nitrification rates
Rates of both gross mineralization and nitrification, as measured by 15N pool dilution, did not change significantly with time since ungulate exclusion in either shrubland or wet forest systems (shrubland P < 0.52 and P < 0.56, wet forest P < 0.54 and P < 0.34, for mineralization and nitrification, respectively; Figs. 2c, and 4b, c). However, when the wet forest data were combined into broad categories of either presence or exclusion of ungulates, a significant increase in gross mineralization was observed in the excluded plots (P < 0.001, Fig. 4b). In the dry forest, gross nitrification decreased significantly by around half (from 3.0 ± 0.4 to 1.4 ± 0.3 mg N kg−1 d−1) over 12 years of exclusion (P < 0.02, Fig. 3c), and gross mineralization showed a saturating response after exclusion was introduced (Fig. 3c).
Resin bag NH 4 + and NO 3 −
Total resin-available inorganic N (NH4+ and NO3−), measured over the course of three to five months, did not vary significantly with increasing time since ungulate exclusion in any of the three studied ecosystems (shrubland P < 0.40, dry forest P < 0.19, wet forest P < 0.30; Figs. 2d, 3d, and 4d). This was also true when NH4+ and NO3− were considered separately (data not shown).
Nitrogen leaching
In the dry forest, total inorganic N leaching through the soil profile increased significantly with increasing time since ungulate exclusion (P < 0.03, r2 = 0.25), from an average of 3.2 mg N cm−2 d−1 in unexcluded plots to 12.4 mg N cm−2 d−1 12 years post-exclusion (Fig. 3e). When examined separately, this increase was significant for NH4+ but not for NO3− (P < 0.001 and P < 0.13, respectively). There was no significant change with time since exclusion in either the dry shrubland or the wet forest (P < 0.26 and P < 0.33, respectively; Figs. 2a, and 4a). Ungulate removal had no impact on soil bulk density in the shrubland or dry forest (Figs. S1 and S2).
Plant foliar δ 15 N
Several plant species showed long-term changes in foliar δ15N (an integrated measure of N availability) with increasing time since ungulate exclusion. In the shrubland, two of the three species examined showed a significant decline between 1 and 10 years post-exclusion; foliar δ15N of herb S. madagascariensis declined by on average 1.5‰ (P < 0.04) while that of shrub D. viscoa declined by 1.1‰ (P < 0.0013; Fig. 1f). In the dry forest, foliar δ15N for S. madagascariensis also declined significantly with time since exclusion, by an average of 1.3‰ over 12 years of ungulate exclusion (P < 0.02; Fig. 3f) while other species showed no significant response. In the wet forest, foliar δ15N was significantly lower across all three species in ungulate-excluded plots (P < 0.0001, P < 0.0001, P < 0.001; Fig. 4f). Of these, foliar δ15N declined significantly with time in two of the three species examined (Fig. 4f). Tree fern C. glaucum declined on average by 2.1‰ (P < 0.0305) and shrub B. argute by 2.2‰ (P < 0.03) over 24 years of ungulate exclusion (Fig. 4f).
Discussion
Overall, we found mixed evidence for N cycling responses to invasive ungulate exclusion across diverse Hawaiian forests and shrublands over periods of 10 to 25 years, adding to a pattern of highly variable responses observed globally (e.g., Siemann et al. 2009; Cushman et al. 2004; Tierny and Cushman 2006). Declines in time-integrated foliar N isotope ratios across multiple species in all three systems suggest systematic changes in N cycling, and likely ecosystem losses of N, but such changes were rarely detected in multiple shorter-term metrics of N availability and cycling (such as inorganic N pool size, leaching, gross mineralization/nitrification, or N2O flux). This contrasting response pattern likely reflects the fact that that assessing ecosystem-level effects of ungulate disturbance is complicated by the spatially and temporally patchy nature of their impacts (Cushman et al. 2004; Long et al. 2017). In this case, variability may also reflect the different disturbance types and intensities of the different ungulates (sheep/goats vs pigs). Temporal variability in these short-term direct measures may also have obscured patterns, given that we prioritized broad sampling across multiple chronosequences and N metrics over extensive temporal replication. Alternatively, the lack of strong responses in many metrics of N cycling may indicate that ungulates do not significantly impact biogeochemical functioning in the first place, though this seems unlikely given the severity of disturbance observed. It is also possible that ungulates (with or without other ongoing disturbances) may have pushed the systems into new stable states that are maintained even after exclusion, or that biogeochemical recovery may operate on time scales longer than 10–25 years.
Foliar N stable isotope analyses offer arguably the most integrated measure of soil N cycle status because they capture the broad changes that result from disturbance over longer time periods (Robinson 2001). Compared with targeted, short-term measurements that capture N pools and fluxes over a period of hours (N2O fluxes), days (mineralization/nitrification assays), or months (resin bags), foliar isotope values reflect a mix of available N in soil (ammonium, nitrate, and potentially amino acids) assimilated by a plant over one or more growing seasons (Robinson 2001). Thus, changes to foliar N isotopes may reflect a ‘smoothed’ long-term signal that is not apparent in short-term measures characterized by higher variability. The observed isotopic decline is consistent with two processes– reduced fractionation of N prior to uptake or greater inputs of fixed N– of which the former is more likely in this context. A lower N isotope ratio in leaves can result from a decrease in the proportion of N that passes through fractionating processes, because N gas losses for example favor the lighter isotope, leaving the remaining soil pool more enriched (Craine et al. 2015). When total available N is exposed to a reduced number of fractionation events, relatively more lighter N is retained in an ecosystem; in this case, the system appears to be less ‘leaky’. In all three systems, we observed a trend (only significant in the dry forest) toward increased rates of mineralization (a process associated with low N isotopic fractionation, Craine et al. 2015) but decreased nitrification (more fractionating, and with potential N gas loss), which can shift the soil inorganic N pool toward ammonium (less mobile and soluble than nitrate). This is consistent with the isotopic results, though not with the resin-bag measurements which integrate soil inorganic N concentrations over 3–5 months. While we did not measure N fixation, the significant increases in isotopically-light N inputs required to drive the relatively large shift in N isotopic values (1.1–2.2 ‰ in non-fixing species) we observed are unlikely. While the native N-fixing tree Acacia koa is known to be highly facultative and can upregulate N fixation in response to demand, their numbers did not change with ungulate exclusion, and invasive fixers such as Morella faya were not common.
Globally, studies examining impacts of ungulate disturbance on biogeochemical functioning report findings that range from substantial to undetectable, with significance varying across metrics and environmental contexts. Most studies have focused on invasive pigs in grasslands, woodlands, and temperate forests. In Mediterranean grasslands, four years of pig exclusion had no apparent impact on N mineralization or nitrification (Cushman et al. 2004), but as is common in such studies, detecting differences may have been obscured by 20 years of extensive disturbance (both pigs and invasive plant species) that likely changed the biogeochemical baseline. Where vegetation changes were observed following pig exclusion, they were attributed to physical changes such as light and removal of competitors, rather than to biogeochemical functioning directly. Significant responses appear to be more commonly detected in forests; for example, Singer et al. (1984) reported pig rooting in temperate forests appeared to increase soluble N in soil, lysimeter soil water, and streamwater by 50–100%. Vitousek (1984) also reported significantly greater N mineralization outside of pig-exclosures in tropical forests. It is, however, difficult to conclude that pigs consistently alter N dynamics from these prior studies because results come from different geographies, ecosystem types, disturbance intensity, duration of impact and recovery, and highly variable methodologies.
Ungulate impacts rarely occur in isolation, and biogeochemical trajectories following their removal are likely to depend on interactions between multiple disturbance types with varying timing and severity. Studies have found differences in how native and invasive species recover from ungulate disturbance, with exotic taxa showing rapid colonization after disturbance in coastal grasslands (Cushman et al. 2004). In Hawaiian woodlands, invasive grasses (that persist after ungulate removal) have been shown to independently alter both the magnitude and seasonal patterns of N mineralization (Mack and D’Antonio 2003). Tierney and Cushman (2006) also suggest that some systems may vary in their rate of recovery based on factors such as rainfall and productivity. We observed the strongest changes in foliar isotopes in the longest exclusion chronosequence in the wet forest. This might reflect a greater time since disturbance (25 years, compared with 10 years in the shrubland), or higher productivity allowing faster vegetation responses. A study in a productive Australian tropical forest found that seedling density and leaf litter cover had recovered to levels observed in long-term pig free plots within 12 years following removal (Taylor et al. 2011). Dry forest and shrubland systems in Hawaiʻi are also vulnerable to fire, which has increased strongly in the past century (Trauernicht et al. 2015) associated with increased fuel loads from invasion of non-native grasses (Mack and D’Antonio 1998, 2003) and long-term trends of increasing drought frequency, severity, and duration (Frazier et al. 2022). These changes have co-occurred with expanding populations of feral ungulates (Wehr et al. 2018). We do not have access to fire history needed to examine the combined and independent effects of fire, invasion, and ungulates in any of the studied chronosequences. However, fire can reduce overall ecosystem N availability through combustion losses (Wong et al. 2019), while ungulates can reduce fire risk by lowering the fine fuel biomass associated in these dry forest systems (Evans et al. 2015; Zhu et al. 2021). The interaction of these disturbance agents can potentially be seen as offsetting, with spatial and temporal variability in their impacts creating complex biogeochemical patterns across the landscape. Further interactions with climate change, climate variability, and drought would only enhance this complexity.
We conclude that exclusion of non-native ungulates in three Hawaiian ecosystems can lead to systematic changes in N availability (as indicated by long-term shifts in foliar isotopic composition). However, direct measures of the major controls on ecosystem nutrient availability (e.g., leaching of inorganic N and gaseous losses) are variable and do not show consistent evidence of detectable change with time since ungulate exclusion, potentially reflecting the difficulty of capturing spatially and temporally heterogenous effects. Despite the widespread acceptance of ungulate removal as a critical practice for restoration of native plant communities, lack of responsiveness of some aspects of soil N availability cycling may in turn help explain why restoration efforts are often slow to manifest (Cole and Litton 2013). The persistence of non-native plant species in ungulate removal plots may either be explained by, or potentially reinforce, ongoing elevated N availability, especially immediately following removal. Future research that explicitly investigates multiple interacting disturbances specific to the ecosystem type may be one avenue for teasing apart the mechanisms of N cycling responses to nonnative ungulate removal, and establishing whether multiple intervention types may be required to increase restoration efficacy.
Data availability
The datasets generated during and analysed during the current study are available in the Knowledge Network for Biocomplexity repository (https://doi.org/10.5063/F13R0RBJ).
References
Allison SD, Vitousek PM (2004) Rapid nutrient cycling in leaf litter from invasive plants in Hawai’i. Oecologia 141:612–619
Barrios-Garcia MN, Ballari SA (2012) Impact of wild boar (Sus scrofa) in its introduced and native range: a review. Biol Invasions 14:2283–2300
Binkley D, Matson P (1983) Ion exchange resin bag method for assessing forest soil nitrogen availability. Soil Sci Soc Am J 47:1050–1052
Bruland GL, Browning CA, Evensen CI (2010) Effects of feral pigs (Sus scrofa) on watershed health in Hawai’i: a literature review and preliminary results on runoff and erosion. In: Roumasset JA, Burnett KM, Balisacan AM (eds) Sustainability science for watershed landscapes. ISEAS Publishing, Singapore, pp 251–278
Bueno CG, Azorín J, Gómez-García D, Alados CL, Badía D (2013) Occurrence and intensity of wild boar disturbances, effects on the physical and chemical soil properties of alpine grasslands. Plant Soil 373:243–256
Cabin RJ, Weller SG, Lorence DH, Flynn TW, Sakai AK, Sandquist D, Hadway LJ (2000) Effects of long-term ungulate exclusion and recent alien species control on the preservation and restoration of a hawaiian tropical dry forest. Conserv Biol 14:439–453
Campbell TA, Long DB (2009) Feral swine damage and damage management in forested ecosystems. For Ecol Manage 257:2319–2326
Chynoweth MW, Litton CM, Lepcxyk CA, Hess SC, Cordell S (2013) Biology and impacts of Pacific Island invasive species. 9. Capra hircus, the Feral Goat (Mammalia: Bovidae). Pac Sci 67:141–156
Cole RJ, Litton CM (2013) Vegetation response to removal of non-native feral pigs from Hawaiian tropical montane wet forest. Biol Invasions 16:125–140
Cole RJ, Litton CM, Koontz MJ, Loh RK (2012) Vegetation recovery 16 years after feral pig removal from a wet Hawaiian forest. Biotropica 44:463–471
Cole RJ, Soper FM, Litton CM, Knauf AE, Sparks K, Gerow KG, Giardina CP, Sparks JP (2021) Restoration benefits of soil nutrient manipulation and weeding in invaded dry and wet tropical ecosystems in Hawai’i. Restor Ecol 29:e13390
Craine JM, Brookshire ENJ, Cramer MD, Hasselquist NJ, Koba K, Marin-Piorra E, Wang L (2015) Ecological interpretations of nitrogen isotope ratios of terrestrial plants and soils. Plant Soil 396:1–26
Cuevas MF, Mastrantonio L, Ojeda RA, Jaksic F (2012) Effects of wild boar disturbance on vegetation and soil properties in the Monte Desert, Argentina. Mamm Biol 77:299–306
Cushman JH, Tierney TA, Hinds JM (2004) Variable effects of feral pig disturbances on native and exotic plants in a California grassland. Ecol Appl 14:1746–1756
Daehler CC (2003) Performance comparisons of co-occurring native and alien invasive plants: implications for conservation and restoration. Annu Rev Ecol Evol Syst 34:183–211
Ellsworth LM, Litton CM, Dale AP, Miura T (2014) Invasive grasses change landscape structure and fire behaviour in Hawaii. Appl Veg Sci 17:680–689
Evans R (2001) Physiological mechanisms influencing plant nitrogen isotope composition. Trends Plant Sci 6:1360–1385
Evans EW, Ellsworth LM, Litton CM (2015) Impact of grazing on fine fuels and potential wildfire behavior in a non-native tropical grassland. Pac Conserv Biol 21:126–132
Fortini LB et al (2020) Local to landscape-level controls of water fluxes through Hawaiian forests: effects of invasive animals and plants on soil infiltration capacity across substrate and moisture gradients. Technical Report HCSU-095, Hawaii Cooperative Studies Unit, pp 1–93
Frazier AG, Giardina CP, Giambelluca TW, Brewington L, Chen, Y.-.L. et al (2022) A century of drought in Hawai’i: geospatial analysis and synthesis across hydrological, ecological and socioeconomic scales. Sustainability 19:12023
Funk JL (2008) Differences in plasticity between invasive and native plants from a low resource environment. J Ecol 96:1162–1173
Giambelluca TW, Chen Q, Frazier AG, Chen Y-L, Chu P-S, Eischeid JK, Delparte DM (2013) Online rainfall atlas of Hawai’i. American Meteorological Society
Gizicki ZS, Tamez V, Galanopoulou AP, Avramidis P, Foufopoulos J (2017) Long-term effects of feral goats (Capra hircus) on Mediterranean island communities: results from whole island manipulations. Biol Invasions 20:1537–1552
Hart SC, Nason GE, Myrold DD, Perry DA (1994a) Dynamics of gross nitrogen transformations in an old-growth forest: the carbon connection. Ecology 75:880–891
Hart SC, Stark JM, Davidson EA, Firestone MK (1994b) Nitrogen mineralization, immobilization, and nitrification. Methods of soil analysis part microbiological and biochemical properties, SSSA Book Series, Soil Science Society of America, pp 985–1018
Hata K, Kohri M, Morita S, Hiradate S, Ecosystems NK (2014) Complex interrelationships among aboveground biomass, soil chemical properties, and events caused by feral goats and their eradication in a grassland ecosystem. Ecosystems 17:1082–1094
Hess SC (2016) A tour de force by Hawaii’s invasive mammals: establishment, takeover, and ecosystem restoration through eradication. Mammal Study 41:47–60
Hess S, Van Vuren D, Whitmer G (2017) Feral goats and sheep. Ecology and management of terrestrial vertebrate invasive species in the United States, CRC Press, Boca Raton, pp 311–332
Knauf AE, Litton CM, Cole RJ, Sparks JP, Giardina CP, Gerow KG et al (2021) Nutrient-use strategy and not competition determines native and invasive species response to changes in soil nutrient availability. Restor Ecol 29:e13374
Long MS, Litton CM, Giardina CP, Deenik J, Cole RJ, Sparks JP (2017) Impact of nonnative feral pig removal on soil structure and nutrient availability in Hawaiian tropical montane wet forests. Biol Invasions 19:749–763
Mack MC, D’Antonio CM (1998) Impacts of biological invasions on disturbance regimes. Science 13:195–198
Mack MC, D’Antonio CM (2003) Exotic grasses alter controls over soil nitrogen dynamics in a Hawaiian woodland. Ecol Appl 13:154–166
McLauchlan KK, Craine JM, Oswald WW, Leavitt PR, Likens GE (2007) Changes in nitrogen cycling during the past century in a northern hardwood forest. Proc Natl Acad Sci USA 104:7466–7470
Murphy MJ, Inman-Narahari F, Ostertag R, Litton CM (2013) Invasive feral pigs impact native tree ferns and woody seedlings in Hawaiian forest. Biol Invasions 16:63–71
Nogueira-Filho SLG, Nogueira SSC, Fragoso JMV (2009) Ecological impacts of feral pigs in the Hawaiian Islands. Biodivers Conserv 18:3677–3683
Ortíz-Alcaraz A, Maya-Delgado Y, Cortés-Calva P, Aguirre-Muñoz A, Rojas-Mayoral E, Cordoba-Matson MV, Ortega-Rubio A (2016) Recovery of vegetation cover and soil after the removal of sheep in Socorro Island, Mexico. Forests 7:91
Ostertag R, Verville JH (2002) Fertilization with nitrogen and phosphorus increases abundance of non-native species in Hawaiian montane forests. Plant Ecol 162:77–90
Peh KSH, Balmford A, Birch JC, Brown C, Butchart SHM, Daley J, Dawson J, Gray G, Hughes FMR, Mendes S, Millett J, Stattersfield AJ, Thomas DHL, Walpole M, Bradbury RB (2015) Potential impact of invasive alien species on ecosystem services provided by a tropical forested ecosystem: a case study from Montserrat. Biol Invasions 17:461–475
Penuelas J, Sardans J, Llusia J, Owen SM, Carnicer J, Giambelluca TW, Rezende EL, Waite M, Niinemets Ü (2009) Faster returns on “leaf economics” and different biogeochemical niche in invasive compared with native plant species. Glob Change Biol 16:2171–2185
Robinson D (2001) δ15N as an integrator of the nitrogen cycle. Trends Ecol Evol 16:153–162
Scowcroft PG, Giffin JG (1983) Feral herbivores suppress mamani and other browse species on Mauna Kea. Hawaii Journal of Range Management 36:638–645
Scowcroft PG, Sakai HF (1983) Impact of feral herbivores on mamani forests of Mauna Kea, Hawaii: bark stripping and diameter class structure. J Range Manag 36:495–498
Siemann E, Carrillo JA, Gabler CA, Zipp R, Rogers W (2009) Experimental test of the impacts of feral hogs on forest dynamics and processes in the southeastern US. Appl Soil Ecol 258:546–553
Singer FJ, Swank WT, Clebsch EEC (1984) Effects of wild pig rooting in a deciduous forest. J Wildl Manag 48:464–473
Spear D, Chown SL (2009) Non-indigenous ungulates as a threat to biodiversity. J Zool 279:1–17
Stephan K, Kavanagh KL (2009) Suitability of the diffusion method for natural abundance nitrogen-15 analysis. Soil Sci Soc Am J 73(1):293–302. https://doi.org/10.2136/sssaj2007.0079
Subler S, Blair JM, Edwards C (1995) Using anion-exchange membranes to measure soil nitrate availability and net nitrification. Appl Soil Ecol 27:911–917
Sullivan BW, Nifong RL, Nasto MK, Alvarez-Clare S, Dencker CM, Soper FM, Shoemaker KT, Ishida FY, Zaragoza Castells J, Davidson EA, Cleveland CC (2019) Biogeochemical recuperation of lowland tropical forest during succession. Ecology 100:e02641
Taylor DL, Leung LK-P, Gordon J (2011) The impact of feral pigs (Sus scrofa) on an Australian lowland tropical forest. Wildl Res 38:437–445
Tierney TA, Cushman JH (2006) Temporal changes in native and exotic vegetation and soil characteristics following disturbances by feral pigs in a California grassland. Biol Invasions 8:1073–1089
Trauernicht C, Pickett E, Giardina CP, Litton CM, Cordell S, Beavers A (2015) The contemporary scale and context of wildfire in Hawai’i. Pac Sci 69:427–444
Vitousek PM (1984) Biological invasions and ecosystem properties: can species make a difference? In: Mooney HA, Drake JA (eds) Ecology of biological invasions in North America and Hawaii. Springer-Verlag, Berlin, pp 163–173
Vitousek PM, Gosz JR, Grier CC, Melillo JM, Reiners WA, Todd RL (1979) Nitrate losses from disturbed ecosystems. Science 204:469–474
Wardle DA, Peltzer DA (2017) Impacts of invasive biota in forest ecosystems in an aboveground–belowground context. Biol Invasions 19:3301–3316
Wehr NH, Hess SC, Litton CM (2018) Biology and impacts of Pacific Islands invasive species. 14. Sus scrofa, the Feral Pig (Artiodactyla: Suidae). Pac Sci 72:177–198
Wehr NH, Kinney KM, Nguyen NH et al (2019) Changes in soil bacterial community diversity following the removal of invasive feral pigs from a Hawaiian tropical montane wet forest. Science Reports 9:14681
Wirthner S, Schütz M, Page-Dumroese DS, Busse MD, Kirchner JW, Risch AC (2012) Do changes in soil properties after rooting by wild boars (Sus scrofa) affect understory vegetation in Swiss hardwood forests? Can J for Res 42:585–592
Wong MY, Neill C, Marino R, Silvério DV, Brando PM, Howarth RW (2019) Biological nitrogen fixation does not replace nitrogen losses after forest fires in the southeastern Amazon. Ecosystems 23:1037–1055
Zhu TR, Litton CM, Giardina CP, Trauernicht C (2021) Moisture availability and ecological restoration limit fine fuels and modelled wildfire intensity following non-native ungulate removal in Hawaii. J Appl Ecol 58:2207–2219
Acknowledgements
We thank Pōhakuloa Training Area-U.S. Army Garrison Hawaiʻi, Hawaiʻi Volcanoes National Park, and the State of Hawaiʻi, Department of Land and Natural Resources, Natural Areas Reserve System for access to research sites and logistical assistance. This work was supported by the DoD Strategic Environmental Research and Development Program (RC-2433), and the USDA-NIFA Hatch (HAW01127-H) and McIntire-Stennis (HAW01123-M) Programs via the College of Tropical Agriculture and Human Resources at the University of Hawaiʻi at Mānoa. We particularly thank Makalani Pina and Taylor Tomita, as well as Nathanael Friday, Kalena Shiroma and Brendan Kote for tireless field and laboratory assistance, and Caroline Dallstream, Mia Marcellus, and Ana Avila Vittorino for feedback on the manuscript.
Funding
This work was supported by the DoD Strategic Environmental Research and Development Program (RC-2433), and the USDA-NIFA Hatch (HAW01127-H) and McIntire-Stennis (HAW01123-M) Programs via the College of Tropical Agriculture and Human Resources at the University of Hawai’i at Mānoa.
Author information
Authors and Affiliations
Contributions
JPS, CPG, CML, and RJC conceived of and designed the study. JPS, KLS, RJC and FMS collected and analyzed samples. FMS and KLS analyzed the data and performed statistical analyses. All authors interpreted results and contributed to the MS, writing led by FMS and JPS.
Corresponding author
Ethics declarations
Conflict of interest
None declared.
Additional information
Responsible Editor: Karsten Kalbitz.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Soper, F.M., Sparks, K.S., Cole, R.J. et al. Inconsistent recovery of nitrogen cycling after feral ungulate removal across three tropical island ecosystems. Biogeochemistry 167, 651–663 (2024). https://doi.org/10.1007/s10533-024-01120-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-024-01120-8