Abstract
The extensive agricultural feedlot operations in the Neuse River Watershed (NRW) in North Carolina result in high nutrient loading, particularly of ammonium (NH4+). In September 2018, Hurricane Florence devastated large portions of the NRW, creating a unique opportunity to study the impact of such hydrological events on the biogeochemistry of riverine and riparian sediments. The high NH4+ concentrations, naturally acidic conditions, and elevated levels of ferric iron [Fe(III)] in Neuse River sediments and soils provide an ideal environment for Acidimicrobium sp. A6 (referred to hereon as A6), a bacterium capable of conducting the Feammox process in which NH4+ is oxidized while iron is reduced. A6 was observed in all sediment samples obtained from the Neuse River, and it is therefore predicted that this process may be an important mechanism for NH4+ removal in this river system. Incubations of NRW samples indicate that the NH4+ oxidation potential via the Feammox process in the NRW is comparable with aerobic NH4+ oxidation by heterotrophic microorganisms. Given the high demand for Fe(III) by the Feammox process, it has been unclear how such a process may occur in sedimentary environments where ferric iron [Fe(III)] might be depleted. The results presented here show that a major hydrologic storm event can result in an increase in Fe(III) and in an increase in the abundance of Fe-reducing bacteria, including Acidimicrobium sp. A6. These findings indicate that major hydrologic storm events may, via the delivery of Fe(III), be capable of enhancing Feammox activity in riverine sediments that favor the Feammox process.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The nitrogen (N) cycle has been greatly impacted by anthropogenic activity (Galloway et al. 2004). With the substantial increase in industrial fertilizer application due to the invention of the Haber–Bosch process, inland and coastal aquatic systems have been drastically altered by increased nutrient loads, causing eutrophication and hypoxia (Galloway et al. 2004). There are several processes that remove reactive N from aquatic and riparian systems, including, but not limited to, biomass sequestration and microbial nitrification followed by denitrification (Bowden 1987; Comín et al. 1997). Microbial nitrification and then denitrification pathways can permanently remove reactive N from aquatic systems and are therefore important for environmental remediation efforts. NH4+ oxidation, the first step of nitrification, can be performed under aerobic conditions by a wide variety of both Ammonia-Oxidizing Bacteria (AOB) and Archaea (AOA), as well as under anaerobic conditions by the Anammox [oxidation of NH4+ by nitrite (NO2−)] and Feammox processes (Jetten 2008; Huang et al. 2018).
The Feammox process, in which NH4+ is oxidized simultaneously with Fe(III) reduction (Clément et al. 2005, Huang and Jaffé 2015; Sawayama 2006; Shrestha et al. 2009, Yang et al. 2012), has been found to contribute to the N cycle in a variety of environmental systems, including forested wetlands, rainforest soils and Chinese paddy soils (Yang et al. 2012, Huang et al. 2016; Ding et al. 2014). Acidimicrobium sp. A6 (referred to here on as A6) has been shown to perform the Feammox process under anaerobic [dissolved oxygen (DO) < 0.02 mg L−1], acidic, and iron (Fe)-reducing conditions (Huang and Jaffé 2015; 2018; Huang et al. 2016). Since inundated sediments are typically anoxic, the Anammox and Feammox processes may be essential for the removal of NH4+ from these sediments.
Since 1990, the state of North Carolina has one of the highest concentrations of hog operations in the United States (USDA), with a large proportion of statewide hog farming located in the lower NRW (map available: NC DEQ, et al.). These concentrated animal feeding operations (CAFOs) produce vast amounts of waste, which is contained in catchment pools. This waste is required to be treated and is then frequently used as fertilizers in adjacent “sprayfields” that cultivate a variety of crops. As a result of subsurface leaching of waste from cesspools and surface run-off from both cesspools and sprayfields, high levels of N loading into the watershed (Brown et al. 2020; Cahoon et al. 1999; Mallin and Cahoon 2003) combined with generally anoxic, acidic, and Fe-rich soils and sediments in the Neuse River riverine and riparian zones provide environmental conditions that may result in a system that is ideal for the Feammox process (Huang et al. 2016).
On September 14, 2018, the NRW was heavily impacted by Hurricane Florence, resulting in 2.4–3.4 m of storm surge, up to 50 cm of precipitation, and winds of 148 km per hour at the point of landfall (Stewart and Berg 2019). Environmental disturbance of this magnitude can greatly impact the microbial community and geochemical profile of the system, and several studies have focused on the effect of major storms on water quality parameters (Fogel et al. 1999, Kan 2018, Mallin et al. 1999; Paerl et al. 2001, 2018; Statham 2012; Voynova et al. 2015; Xu et al. 2017). Results of these studies are varied, not only because of the very different geographical settings, but also because given the challenges of sampling during a major storm, especially a hurricane/typhoon, some sampling studies were conducted during a storm event, while others occurred after the storm event.
Fong et al. (2020) reported increased concentrations of phosphorous (P) as well as nitrate (NO3−) and NO2− right after a major 10-day rain event in the near shore environment in French Polynesia. Zhang et al. (2022), reported that the particulate P load in a subtropical estuary during a storm more than doubled, to a large degree because of oxidation of Fe(II) to Fe(III) in surficial sediments which sorbed dissolved P, indicating that that reduced species can be oxidized during storm events. These findings are consistent with those reported by Huang et al. (2021), who reported changes in concentrations of N, sulfur (S), and P compounds in sediments of the Pearl River and Zhanjiang estuaries one month after a typhoon event. Changes between pre- and post-storm showed increased concentrations in sediments of some factors (i.e., DO, total organic carbon (TOC), Fe(III), N species], no effect on other factors (i.e., salinity), and decreased concentration of some reduced species [i.e., Fe(II), sulfite (S2−)]. During the period studied (2016–2018), these estuaries were impacted by a typhoon every year and during that period, pre-storm conditions were reestablished within a year prior to the next disturbance. A study focusing on the impacts of Hurricane Harvey on the water quality of three Texas estuaries, showed that salinity increased during the storm and returned to pre-storm conditions within a month. There was an increase in inorganic nutrients, similar as observed for the Pearl River and Zhanjiang estuaries described above, which returned to pre-storm conditions by the winter (Walker et al. 2021). In the Pearl River and Zhanjiang estuaries, shifts in nutrient concentration showed significant correlations with changes in microbial abundance of certain genera, including the Acidimicrobium genus to which A6 belongs. Prompted by these results, further study of impacts from storm disturbance on the Feammox process in the nutrient-rich ecosystem of the NRW was pursued.
This study aims to (1) assess the impact of Hurricane Florence on the geochemistry and microbial community of the NRW through spatiotemporal analysis, (2) investigate the specific effect of storm disturbance on the presence and abundance of the Feammox microorganism (A6) through qPCR and Illumina sequencing, and (3) determine the possible role of the Feammox process in the overall oxidation of NH4+ in riparian sediments of the NRW through laboratory incubation analysis. This study lends insight into the role of the Feammox process on NH4+ oxidation in the NRW, as well as the impact of storm disturbance on the Feammox process and the broader microbial community.
Methods and materials
Site details
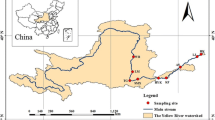
Four sampling sites were chosen along the NRW (Fig. 1) and are labeled A-D by their location along the watershed, with the farthest upstream site labeled “A.” Sites were sampled by 2–3 transects per site, and the size of the river at each transect is described in Table 1 (determined using USGS StreamStats database, with river characterization according to Higgins et al. 2005). The sampling sites can also be described based on the degree of pollution they receive, predicted by the proximity of the sampling site to nearby CAFOs, as shown in Fig. 1. CAFO locations throughout North Carolina can be viewed through the North Carolina Department of Environmental Quality digital map (NC DEQ, et al.). Transect location and distance was selected based on where a tributary of the Neuse was located near to an identified CAFO and near the main stem of the stream.
Field sampling
Field sampling was conducted at three time points: June 2018, October 2018, and June 2019. These sampling times varied substantially due to the occurrence of Hurricane Florence on September 14, 2018. The sample points may also be referred to as “pre-storm”, “recent post-storm”, and “late post-storm”, respectively. Typical instantaneous stream flow values in June at site C may range from 20 to 40 m3/sec, whereas comparable metrics during and following Hurricane Florence in October 2018 were measured between 650 and 850 m3/sec.
A 4 m transect was established at each sampling site. One end of the transect was established at a distance of 50 cm into the stream, ensuring that this initial sampling point was fully submerged. Each transect was then run perpendicular to the river flow direction up the riverbank, through the riparian zone. Each site was sampled along this transect at 5 equidistant points, with 1 m of separation between each sampling point. With a total of 9 transects throughout the watershed, this sampling process resulted in a total of 45 sampling points. Notation to describe each sampling site is hereon referred to as “Xa,b” with X = site ID (A-D), a = transect number (1–3), and b = the location along the transect (1–5; increments of 1 m, with the origin 50 cm into the stream bed).
At the origin point of each transect, pH was measured using an Ecosense® pH meter (Model no.: pH10). Two sets of water samples were also taken at the transect origin point, one of which was acidified with 1N hydrochloric acid (HCl), while the other was passed through a 0.2 µm filter. For sediment/soil collection, at each of the 45 sampling points, cores were obtained to a depth of 30 cm using a manual corer (2.5 cm diameter). Sediment dry weight and pH were obtained through laboratory analysis of sediment cores post-sampling. Finally, additional sediment and water samples were collected at the same locations and acidified with concentrated HCl and 1 M sulfuric acid (H2SO4) for analysis of Fe(II) and NH4+ concentrations, respectively. All samples were refrigerated at 4 °C for analysis in the laboratory; all analyses were conducted within one month of sampling.
Feammox incubations
To assess the potential for NH4+ oxidation pathways in the sediments (i.e., aerobic via AOA or AOB, or anaerobic via Anammox or Feammox), a series of laboratory incubations were conducted under different conditions. Because the goal was to assess the potential for NH4+ oxidation and not actual field rates, incubations with sediment from NRW June 2019 field samples were conducted using inorganic Fe-NH4+ medium prepared according to previously described methods (Huang et al. 2016), so as to ensure that the NH4+ and Fe(III) availability was not limiting. To determine the effect of the addition of the inorganic Fe-NH4+ medium, control incubations were also conducted with NRW samples and an amount of deionized (DI) water equivalent to the added medium in the previously described incubations. Sediment samples from each location were divided into 12 × 1-g (air-dried equivalent) subsamples, and added into 25-mL serum vials, with 15 mL medium (or DI water). Incubations were then conducted under three different conditions: (i) oxic, (ii) anoxic, and (iii) anoxic with acetylene. For the anoxic incubations, vials were sealed with butyl rubber stoppers without PTFE liner. The headspace (10 ml) of each vial was vacuumed and then flushed with an ultra-high purity N2/CO2 (80:20) mixture to achieve anoxic conditions. For oxic incubations, vials were covered with Parafilm. During the whole incubation period, DO was approximately 2 mg/L under oxic conditions, and in the anoxic incubation, DO was lower than 0.1 mg/L, the level at which all aerobic NH4+ oxidation is inhibited (Ramanathan et al. 2014).
An acetylene block experiment was conducted to determine the activity of A6 relative to other potential NH4+ oxidizers, as previously described (Huang and Jaffé, 2015). Acetylene does not affect the Feammox process but does block the Anammox process (Jensen et al. 2007) and the aerobic oxidation of NH4+ by AOB and AOA (Offre et al. 2009). Acetylene was added to the headspace to achieve the concentration of 35 μmol/L. In all cases, vials were placed on a shaker at 25 °C. Triplicate samples were collected destructively every 10 days during 40-day incubations for chemical and microbial analysis. For the acetylene block experiment, 5 ml headspace gas was also collected to monitor N2O gas accumulation.
Chemical analysis
NH4+, NO2−, Fe(III), Fe(II) and pH were evaluated in sediment samples, while NH4+, NO2−, NO3−, TOC, and total nitrogen (TN) were evaluated in water column samples. Sediment samples were extracted with 0.5 M HCl. Fe content and species concentrations in the sediment extract were quantified using the ferrozine method developed by Lovley and Phillips (1987) and adapted by Komlos and Jaffé (2004). As previously stated in Sect. “Field sampling”, water samples were taken at each site, with one group of samples acidified using 1N HCl and one group filtered using 0.2 µm filters. Acidified water samples were additionally filtered, and soil extract and water samples (both acidified and non-acidified) were analyzed for inorganic nitrogen [NH4+, NO3−, and NO2−] using a Dionex™ Ion Chromatograph (LC3000) with IonPac CS16 or AS18 column (Dionex, Sunnyvale, CA, USA), and methods described previously (Huang and Jaffé, 2015). Method detection limits were 0.1 mg-N/L for NH4+, NO3−, and NO2− measurement by ion chromatography. TOC and TN were determined using Shimadzu Total Organic Carbon (TOC-VCDN) and Total Nitrogen (TMMI) analyzer.
Microbial analysis
Sediment DNA was extracted from 0.5 g homogenized sediment samples, and DNA from the incubation subsamples was extracted from 0.5 ml slurry, both using the FastDNA® spin kit for soil (MP Biomedicals, US) as detailed by the manufacturer. Purity and DNA concentrations were analyzed using a Nano-drop 2000 spectrophotometer (Thermo Scientific, USA).
The V4 region of the 16S rRNA gene of bacteria was amplified using primer-set 515F-806R (Caporaso et al. 2012), and sequencing was performed on an Illumina Hiseq PE250 platform at Novogene Co. (Beijing, China). A total of 46,090–96,773 qualified reads were generated from 49,588–100,359 raw sequences of the 16S rRNA gene for each sediment sample. A 97% similarity cut-off was used to classify Operational Taxonomic Units (OTUs) (Edgar 2013), and 1080–4711 OTUs were generated from each sample. The representative sequence for each OTU was then screened for further annotation. 16S rRNA gene sequence taxonomy was analyzed with Mothur (version v.1.30.1) against the Silva SSUrRNA database using a confidence threshold of 0.8–1 (Wang et al. 2014). Rarefaction curves were generated for all microbial samples via R, and Observed-species, Chao1, Shannon, Simpson, ACE, and Goods-coverage indices were estimated via Qiime (Version 1.7.0).
Acidimicrobiaceae bacteria were targeted by primer set acm342f/439r (Huang and Jaffé 2015), and quantified via qPCR analysis on a 96-well StepOnePlus Real-Time PCR System (Applied Biosystems, USA). PCR program and specific annealing temperatures were chosen based on previously described methods from Huang et al. 2018. The PCR amplification efficiencies were 85–113%, and correlation coefficients (R2) for all assays were > 0.99.
Statistical analysis
T-tests were used to evaluate statistical differences in chemical data between sites and time points, using a minimum of a 95% significance level. Redundancy analysis (RDA) was used to evaluate the composition of microbial communities constrained by environmental variables using the vegan package (Oksanen et al. 2020).
Results and discussion
Chemical analysis from field sampling
Solutes relevant to the N and Fe cycles were analyzed at all sites for both the water and sediment samples, and NH4+, NO3−, NO2−, TN, and TOC were analyzed in water column samples. Figure 2 displays the results from this analysis; in select charts, location B1 has been removed due to high variability attributed to analytical errors. NO2− was not detected in any water column or sediment samples, though its absence may be attributed to the rapid abiotic reaction of Fe(II) with NO2− in anoxic sediments or the biotic conversion of NO2− to NO3− in oxic environments (Weber et al. 2001; Coby et al. 2011). Removal of NO2− by anammox bacteria was unlikely to play a large role in the absence of NO2− observed. Analyses of the microbial community (see Sect. “Impact of storm disturbance on microorganisms involved in the N and Fe cycles”) showed low abundance of anammox bacteria, and laboratory incubations (see Sect. “Potential role of the Feammox process in NH4+ oxidation in Neuse River riparian sediments”) conducted with and without acetylene did not show a significant difference in NH4+ removal, indicating a lack of anammox activity. Although at some locations there is no noticeable change in concentration, overall, NH4+ and NO3− concentrations were significantly elevated in the pre-storm samples vs. post storm (p < 0.001; p < 0.05), while TN and TOC concentrations were generally low (Fig. 2). There was an observed decrease in NH4+ and NO3− concentrations and an increase in TN and TOC concentrations in recent post-storm samples, with decreases in water column NH4+ attributed to dilution effects and a flushing of NH4+ sequestered in sediments due to the storm.
Sediment pH ranged from 5.3 to 6.4 at all sampled transects in June 2018. Sediment pH changes due to storm disturbance can be loosely described by their river size as characterized in Table 1. Generally, headwater transects (D1, A1 and B1) saw a decrease in mean pH following the storm event (p < 0.001), while the medium river transects (C1 and C2) showed an increase in mean pH from pre- to recent post-storm conditions (p < 0.05) (Fig. S1). However, there was no distinct trend observed in the change in pH values at all sites between recent post-storm and late post-storm sampling time points (Fig S1), as four of the sites (A1, B1, C1, and C2) appear to return to pre-storm conditions, while the other five sites (A2, B2, C3, D1, and D2) do not.
For sediment samples collected in June 2018, the majority of the sampling locations showed levels of NH4+ ranging from 15.9 to 204.3 mg/kg. NH4+ concentrations universally decreased following the storm event in September 2018 (p < 0.05) (Fig. 3; see concentrations for June and October 2018), ranging from a 23–92% decrease in concentration, varying by site. This decrease can be attributed to a scouring of NH4+ from the sediments, the deposition of unpolluted sediments delivered due to the storm event, or the decrease in the NH4+ source through the flushing of CAFO cesspools. These results are also consistent with studies of the effect of land use on nutrient concentrations during storms in sub-catchments of the Hii River Basin, in Western Japan (Ide et al. 2019). This study showed that in forested catchments the nutrient concentrations in streams decrease during major storm events, while the opposite was true for agricultural catchments. While land use in the NRW consists of a mixture of forest, agriculture, and urban environments, the riparian zones that were sampled are predominantly forested (Fig. 1). It is therefore to be expected that trends from the sampled locations would be consistent with trends in the forested catchments of the Hii River Basin.
While the response to storm disturbance was mostly consistent across sampling sites, the degree to which pre-storm conditions were reestablished at these sites varied greatly (Fig. 3). Although the reason for this variability is not clear, it could be attributed to differences in hydraulic profile (stream size) and contamination between the sites (Table 1), as well as differences in the rates of operation recovery of individual CAFOs.
Fe concentrations in the sediment samples were lower in June 2018 (Total Fe = 3.64 ± 1.82 g/kg), however following the storm disturbance, both Fe(III) and Fe(II) increased at nearly all sampled locations and remained elevated in June 2019 (Fig. 4). This increase may be attributed to deposition of sediment transported from upstream sources via erosion. The Fe(III):Fe(II) ratio was assessed for all sampling sites, transects, and time points (Table S1). While there was no significant trend based on sampling time point, there was a clear difference between sites. A ratio of Fe(III):Fe(II) > 1.0 was observed for all sampling locations at both sites A and C, while Fe(III):Fe(II) < 1.0 was observed for most sampling locations at site D. There was no discernable or significant trend for the B transects. Results from these analyses suggest that sediments at sites A and C are more oxidized while sediments at site D are more reduced, however confirmation would require redox potential field data that was not collected during this study.
Impact of storm disturbance on the microbial community of Neuse River sampling sites
The alpha diversity indices of microbial communities in the sediments were compared between sampling sites, as well as between all sediment samples collected at pre- (June 2018), recent post- (October 2018) and late post-storm (June 2019) periods (Table S2). Higher values of Chao1 and Shannon Index were observed in the recent post-storm samples than in pre-storm samples (p = 0.0215; p < 0.001), for all analyzed samples except C1-1 and D2-4, indicating an overall increase in diversity. Studies at White Clay Creek (Chester Co., PA, USA) following Hurricane Irene and Tropical Storm Lee also showed increases in bacterial diversity, which the authors attribute to the large input of nutrients to the river, prompting shifts in the bacterial community structure (Kan. 2018). This nutrient input provides the energy and material essential for microbial growth, resulting in an increase in microbial abundance in an ecosystem. While some studies show that high nutrient inputs, particularly of N, can result in a decrease in bacterial diversity, such decreases are not present in NRW sampling data (Huang et al. 2021; Liu et al. 2020).
The relative abundance of the top 24 phyla (about 73.5 to 81.3% of the total bacterial abundance) for all sampling sites was analyzed, with Proteobacteria analyzed at the class level (Fig. 5). There was an observed shift in the dominant phyla under post-storm conditions, demonstrating that storm events can cause alterations in the overall microbial community structure. This result is consistent with reported observations from other systems impacted by storm disturbance (Huang et al. 2021; Paerl et al. 2018; Steichen et al. 2020; Tseng et al. 2013). The largest shift in the NRW was observed in the relative abundance of Actinobacteria, which was shown to decrease under recent post-storm conditions, and of Acidobacteria, which showed an increase in relative abundance. Both Actinobacteria and Acidobacteria are highly abundant in soils and sediments and are known for their remarkable ability to survive in a wide variety of environmental conditions, including extreme temperature and pH ranges. Thus, they are highly tolerant of environmental turbulence (Fierer 2017; Qin et al. 2019; Kan 2018). For most of the top phyla, the shift observed from pre- to post-storm conditions was not reversed by June 2019, indicating that there is not a recovery effect for the microbial community to pre-storm conditions over that timeframe.
Although the water temperature decreased by 5 °C between June and October, this decrease in temperature is unlikely to explain the shifts in the microbial community. The average water temperature of the Neuse River at the sampling location for June is 29 °C, while for October it is 24 C (U.S. Geological Survey). Bucci et al. 2014, studying seasonal changes in the microbial community structure in sediments of the Neuse River basin during wet and dry seasons from 2007 to 2008, found that temperature changes during the summer season, which varied between 22 and 27 °C did not have a major effect on the microbial community structure. Hence, the temperature shift between June and October observed here is expected to have only a minor effect on the microbial community structure. Microbial community analysis from Huang et al. (2018) shows that while some changes in N-loading and the activity of denitrifying genes (narG and nirS) may occur under much larger seasonal temperature changes (12–24 °C), large shifts in microbial community structure were not observed. In terms of the Feammox process, this decrease in temperature from June to October might have had a small positive effect on the activity of A6, since it was shown in laboratory incubations that the optimal temperature for NH4+ oxidation rate by A6 is approximately 20 °C (Ruiz-Urigüen 2014). Hence, shifts in the microbial community are attributed mostly to the direct effects of the storm.
Impact of storm disturbance on microorganisms involved in the N and Fe cycles
The shift in absolute abundance of genera specific to the N- and Fe-cycles from pre-storm (June 2018) to recent post-storm (October 2018) periods was investigated to determine the impacts of the hurricane on the microbial community (Fig. 6). All Fe-cycling bacteria show a significant increase in absolute abundance (p = 0.0465), including Acidimicrobiia, which is the genus to which the Feammox-conducting bacteria A6 belongs. Finally, several genera were observed under recent post-storm conditions that were not detected in pre-storm conditions, including Shewanella and Alcanivorax, however there were no significant differences between sites for these particular bacteria. This increase or introduction of specific genera may be taken as an indication of sediment deposition due to the storm disturbance. The shift in absolute abundance of these genera was not analyzed between October 2018 and June 2019, due to the longer period of time between sampling points. Field data from this study is not of high enough resolution to account for all of the potential external factors that could cause a shift in the absolute abundance of N-cycling and Fe-reducing bacteria.
The percent change in microbial absolute abundance from pre- (June 2018) to post-storm (October 2018), periods defined as: \(\frac{\left({\mathrm{A}.\mathrm{A}.}_{\mathrm{post}-\mathrm{storm}}\right)-({\mathrm{A}.\mathrm{A}.}_{\mathrm{pre}-\mathrm{storm}})}{{\mathrm{A}.\mathrm{A}.}_{\mathrm{post}-\mathrm{storm}}}\), where “A.A.” refers to absolute abundance. Select data points that indicated concentrations below detection limit were excluded from statistical analysis
In the previously studied Pearl River Estuary, China (Huang et al. 2021), similar shifts in the microbial community were observed, however there was also an observed “recovery” back to pre-storm conditions each year following storm disturbance. This complete recovery was not observed in the NRW, likely due to differences in ecosystem type and the frequency of intense storm disturbance. The Pearl River Estuary experiences annual typhoon events, while the Neuse River is less frequently impacted, allowing for a more established baseline condition. However, given the contaminated nature of the pre-storm NRW, “recovery” may not be a desirable condition and storm disturbance may actually introduce new or expand existing opportunities for environmental processes such as Feammox.
The changes in A6 numbers due to storm disturbance were assessed at 13 sampled points along 8 different transects via qPCR analysis using A6-specific primers, as previously described in Huang et al. 2018. Half of the samples showed an increase in A6 numbers from pre- to recent post-storm conditions, while the other half showed a decrease in A6 numbers post-storm event (October 2018) (Table S3). These increases or decreases in A6 numbers do not group by site location or any other independent variables. It is likely that this variation is due to site specific variations or microenvironments that are not captured in this sampling survey.
Potential role of the Feammox process in NH4 + oxidation in Neuse River riparian sediments
Incubations of select June 2019 field sampling Neuse River sediments reveal active NH4+ oxidation under both oxic (DO = 2 mg/L) and anoxic (DO < 0.1 mg/L) conditions over a 40-day period. Over the first 14 days of incubation, there was no significant difference in the NH4+ removal between incubations under both conditions (p = 0.1337), indicating that for the amended NRW samples, anoxic and oxic NH4+ oxidation processes are comparable in their removal abilities. Comparing the two groups of anaerobic incubations, with or without acetylene, 761.1% and 72.0% of NH4+ was removed over a 40-day incubation, respectively (Fig. 7). Since these results are statistically equal (p = 0.85), it can therefore be concluded that the Feammox process is the main driver of NH4+ oxidation in the Neuse River sediments, as opposed to the Anammox process, which is blocked by acetylene (Jensen et al. 2007).
Analysis of total numbers of A6 for all incubations was conducted, however given the slow doubling time of A6 (10–14 days), there was no consistent trend observed over the 40-day incubations (Fig. 8). Generally, there was no significant difference found between the anaerobic incubations run with and without the addition of acetylene (p = 0.3977), indicating that there was no overt inhibitory or toxic effect of acetylene on the A6 bacteria. Initial A6 numbers for these incubations are provided in Table S3.
Redundancy analysis (RDA) of microbial and geochemical properties across all time points
The biogeochemical changes discussed in this paper are summarized by the redundancy analysis shown in Fig. 9, which shows how site clustering changed over the three sampling dates. The environmental factors that have the greatest impact on the distribution of microorganisms involved in the nitrogen and iron cycles at these sampling locations are NH4+, Fe(II), Fe(III), and pH are. Although the TOC exhibits the largest changes from pre- to post-storm conditions, its influence on these microbial communities is limited (not shown in Fig. 9). Furthermore, in terms of the Feammox-conducting bacterium A6, which is an autotroph, changes in TOC are not expected to have a direct impact on the Feammox process.
Redundancy analysis (RDA) illustrating the effects of environmental factors (arrows) on microbial community structure (symbols) in samples from a June 2018, b October 2018, and c June 2019. The values of axes 1 and 2 are the percentage that can be explained by the corresponding axis. Data points are from different sampling locations (A-D)
The results of the RDA indicate that NH4+ has the greatest influence on the microbial community at site D, while pH had the largest influence on the microbial community at site B. In June 2018 (pre-storm), individual sites are clustered together with noticeable differentiation between individual sites. By October 2018 (recent post-storm), this clustering by site is no longer observed, suggesting a homogenization of the microbial and geochemical factors. Finally, by June 2019 (late post-storm), it is possible to differentiate again between some of the individual sites, however, as discussed in Sect. “Impact of storm disturbance on microorganisms involved in the N and Fe cycles”, a complete recovery to the same degree of clustering as noted in the pre-storm time point is not observed at that point in time.
Based on the results shown in Fig. 9, one can conclude that the impact of the hurricane at each site (A-D) was a de-clustering (or homogenization) of the microbial community and geochemical composition following the storm, with a partial recovery by June 2019 to the pre-storm conditions.
Conclusion
Analysis of the biogeochemistry of water column and sediment samples from the NRW has revealed the impacts of agricultural activity and major storm disturbance on microorganisms involved in the N and Fe cycles. Shifts in the microbial community observed from pre- to post-storm time periods have likely occurred through dilution, sediment deposition, or shifts in redox processes. These geochemical processes have created new environmental niches within the ecosystem, enabling particular microbial processes to thrive in conditions created by storm disturbance.
Incubation experiments investigating the Feammox process and A6 abundance in Neuse River sediments have revealed promising indications of Feammox viability in agriculture- and storm-impacted ecosystems. These experiments found that the potential for NH4+ removal by microbial oxidation was comparable between aerobic and anaerobic incubations, even under conditions that block Anammox activity, indicating that Feammox is capable of playing a substantial role in the oxidization of NH4+ in this ecosystem. Finally, following the storm event, there was an observed increase in Fe reducers, Fe(III) concentration, and absolute A6 abundance. While the importance of in situ Feammox activity relative to other denitrification processes in the NRW requires further study, results from lab and limited field analysis suggest that the Feammox process in the NRW is enhanced in the wake of major storm disturbance. Further investigation into the impact of such disturbance on the N cycle as a whole, inclusive of the Feammox process, will lend further insight into the biogeochemical cycles at play in riparian sediments.
Data availability
Data generated in this study can be made available upon request from the corresponding author.
References
Bowden WB (1987) The biogeochemistry of nitrogen in Freshwater Wetlands. Biogeochemistry 4(3):313–348
Brown CN, Mallin MA, Loh AN (2020) Tracing nutrient pollution from industrialized animal production in a large coastal watershed. Environ Monit Assess. https://doi.org/10.1007/s10661-020-08433-9
Bucci JP, Szempruch AJ, Caldwell JM, Ellis JC, Levine JF (2014) Seasonal Changes in microbial community structure in freshwater stream sediment in a North Carolina river basin. Diversity 6(1):18–32. https://doi.org/10.3390/d6010018
Cahoon LB, Mikucki JA, Mallin MA (1999) Nitrogen and phosphorus imports to the cape fear and neuse river basins to support intensive livestock-production. Environ Sci Technol 33:410–415. https://doi.org/10.1021/es9805371
Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Huntley J, Fierer N, Owens SM, Betley J, Fraser L, Bauer M, Gormley N, Gilbert JA, Smith G, Knight R (2012) Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J 6:1621–1624. https://doi.org/10.1038/ismej.2012.8
Clément JC, Shrestha J, Ehrenfeld JG, Jaffé PR (2025) Ammonium oxidation coupled to dissimilatory iron reduction under anaerobic conditions in wetland soils. Soil Biol Biochem 37(12):2323–2328. https://doi.org/10.1016/j.soilbio.2005.03.027
Coby AJ, Picardal F, Shelobolina E, Xu HF, Roden EE (2011) Repeated anaerobic microbial redox cycling of iron. Appl Environ Microbiol 77:6036–6042
Comín FA, Romero JA, Astorga V, García C (1997) Nitrogen removal and cycling in restored wetlands used as filters of nutrients for agricultural runoff. Water Sci Technol 35(5):255–261
Ding LJ, An XL, Li S, Zhang GL (2014) Nitrogen loss through anaerobic ammonium oxidation coupled to iron reduction from paddy soils in a chronosequence. Environ Sci Technol 48:10641–10647
Edgar R (2013) UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods 10(10):996–998
Fierer N (2017) Embracing the unknown: disentangling the complexities of the soil microbiome. Nat Rev Microbiol 15(10):579–590. https://doi.org/10.1038/nrmicro.2017.87
Fogel ML, Aguilar C, Cuhel R, Hollander DJ, Willey JD, Paerl HW (1999) Biological and isotopic changes in coastal waters induced by Hurricane Gordon. Limnol Oceanogr 44:1359–1369
Fong CR, Gaynus CJ, Carpenter RC (2020) Extreme rainfall events pulse substantial nutrients and sediments from terrestrial to nearshore coastal communities: a case study from French Polynesia. Sci Rep 10:2955. https://doi.org/10.1038/s41598-020-59807-5
Galloway JN, Dentener FJ, Capone DG, Boyer EW, Howarth RW, Seitzinger SP, Asner GP, Cleveland CC, Green PA, Holland EA, Karl DM, Michaels AF, Porter JH, Townsend AR, Vörösmarty CJ (2004) Nitrogen Cycles: past, present and future. Biogeochemistry 70:153–226
Higgins JV, Bryer MT, Khoury ML, Fitzhugh TW (2005) A freshwater classification approach for biodiversity conservation planning. Conserv Biol 19:432–445. https://doi.org/10.1111/j.1523-1739.2005.00504
Huang S, Jaffé PR (2015) Characterization of incubation experiments and development of an enrichment culture capable of ammonium oxidation under iron-reducing conditions. Biogeosciences 12:769–779
Huang S, Jaffé PR (2018) Isolation and characterization of an ammonium-oxidizing iron reducer: Acidimicrobiaceae sp. A6. PLoS ONE 13(4):e0194007. https://doi.org/10.1371/journal.pone.0194007
Huang S, Chen C, Peng X, Jaffé PR (2016) Environmental factors affecting the presence of Acidimicrobiaceae and ammonium removal under iron-reducing conditions in soil environments. Soil Biol Biochem. https://doi.org/10.1016/j.soilbio.2016.04.012
Huang S, Chen C, Jaffé PR (2018) Seasonal distribution of nitrifiers and denitrifiers in urban river sediments affected by agricultural activities. Sci Total Environ 642:1282–1291. https://doi.org/10.1016/j.scitotenv.2018.06.116
Huang S, Sherman A, Chen C, Jaffé PR (2021) Tropical cyclone effects on water and sediment chemistry and the microbial community in estuarine ecosystems. Environ Pollut. https://doi.org/10.1016/j.envpol.2021.117228
Ide J, Takeda I, Somura H, Mori Y, Sakuno Y, Yone Y, Takahashi E (2019) Impacts of Hydrological changes on nutrient transport from diffuse sources in a rural river basin, Western Japan. J Geophys Res Biogeosci 124(8):2565–2581. https://doi.org/10.1029/2018JG004513
Jensen MM, Thamdrup B, Dalsgaard T (2007) Effect of specific inhibition on anammox and denitrification in marine sediments. Appl Environ Microbiol 73:3151–3158
Jetten M (2008) The microbial nitrogen cycle. Environ Microbiol 10:2903–2909
Kan J (2018) Storm events restructured bacterial community and their biogeochemical potentials. J Geophys Res Biogeosciences 123:2257–2269. https://doi.org/10.1029/2017JG004289
Komlos J, Jaffé PR (2004) Effect of iron bioavailability on dissolved hydrogen concentrations during microbial iron reduction. Biodegradation 15(315):325
Liu W, Jiang L, Yang S, Wang Z, Tian R, Peng Z, Chen Y, Zhang X, Kuang J, Ling N, Wang S, Liu L (2020) Critical transition of soil bacterial diversity and composition triggered by nitrogen enrichment. Ecology. https://doi.org/10.1002/ecy.3053
Lovley DR, Phillips EJP (1987) Competitive mechanisms for inhibition of sulfate reduction and methane production in the zone of ferric iron reduction in sediments. Appl Environ Microbiol 53:2636–2641. https://doi.org/10.1128/aem.53.11.2636-2641.1987
Mallin MA, Cahoon LB (2003) Industrialized animal production—A major source of nutrient and microbial pollution to aquatic ecosystems. Popul Environ 24:369–385. https://doi.org/10.1023/A:1023690824045
Mallin MA, Posey MH, Shank GC, McIver MR, Ensign SH, Alphin TD (1999) Hurricane effects on water quality and benthos in the cape fear watershed: natural and anthropogenic impacts. Ecol Appl 9:350–362
NC DEQ, ESRI, HERE Technologies, National Park Service. DWR Animal Operation Permits. ESRI. https://ncdenr.maps.arcgis.com/apps/webappviewer/index.html?id=85ae6392d0e94010a305eedf06e3f288. Accessed 2019
Offre P, Prosser JI, Nicol GW (2009) Growth of ammonia-oxidizing archaea in soil microcosms is inhibited by acetylene. FEMS Microbiol Ecol 70:99–108
Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, Mcglinn D (2020). vegan: community ecology package. R Package Version 2.5–7. https://CRAN.R-project.org/package=vegan
Paerl HW, Crosswell JR, Van Dam B, Hall NS, Rossignol KL, Osburn CL, Hounshell AG, Sloup RS, Harding LW (2018) Two decades of tropical cyclone impacts on North Carolina’s estuarine carbon, nutrient and phytoplankton dynamics: implications for biogeochemical cycling and water quality in a stormier world. Biogeochemistry 141:307–332. https://doi.org/10.1007/s10533-018-0438-x
Paerl HW, Bales JD, Ausley LW, Buzzelli CP, Crowder LB, Eby LA, Fear JM, Go M, Peierls BL, Richardson TL, Ramus JS (2001) Ecosystem impacts of three sequential hurricanes (Dennis, Floyd, and Irene) on the United States’ largest lagoonal estuary, Pamlico Sound, NC. Proc Natl Acad Sci U S A 98:5655–5660. https://doi.org/10.1073/pnas.101097398
Qin S, Li W, Klenk H, Hozzein W, Ahmed I (2019) Editorial: Actinobacteria in special and extreme habitats: diversity, function roles and environmental adaptations, Second edition. Front Microbiol. https://doi.org/10.3389/fmicb.2019.00944
Ramanathan G, Sales CM, Shieh WK (2014) Simultaneous autotrophic denitrification and nitrification in a low-oxygen reaction environment. Water Sci Technol 70(4):729–735. https://doi.org/10.2166/wst.2014.292
Ruiz-Urigüen MP (2014) Characterizing the Microbial Mediated Feammox Process: Temperature Effect and Electron Acceptors. MSE Thesis, Princeton University
Sawayama S (2006) Possibility of anoxic ferric ammonium oxidation. J Biosci Bioeng 101:70–72
Shrestha J, Rich JJ, Ehrenfeld JG, Jaffé PR (2009) Oxidation of ammonium to nitrite under iron-reducing conditions in wetland soils laboratory, field demonstrations, and push-pull rate determination. Soil Sci 174:156–164
Statham PJ (2012) Nutrients in estuaries—An overview and the potential impacts of climate change. Sci Total Environ 434:213–227. https://doi.org/10.1016/j.scitotenv.2011.09.088
Steichen JL, Labonté JM, Windham R, Hala D, Kaiser K, Setta S, Faulkner PC, Bacosa H, Yan G, Kamalanathan M, Quigg A (2020) Microbial, physical, and chemical changes in Galveston Bay following an extreme flooding event, hurricane Harvey. Front Mar Sci 7:1–21. https://doi.org/10.3389/fmars.2020.00186
Stewart SR, Berg R (2019) National Hurricane Center tropical cyclone report: Hurricane Florence (AL062018). Natl Hurric Cent. pp 1–98
Tseng CH, Chiang PW, Shiah FK, Chen YL, Liou JR, Hsu TC, Maheswararajah S, Saeed I, Halgamuge S, Tang SL (2013) Microbial and viral metagenomes of a subtropical freshwater reservoir subject to climatic disturbances. ISME J 7:2374–2386. https://doi.org/10.1038/ismej.2013.118
U.S. Department of Agriculture, Economic Research Service. “Sector at a Glance: Hog Production.” https://www.ers.usda.gov/topics/animal-products/hogs-pork/sector-at-a-glance/. Accessed 2019
U.S. Geological Survey (2001) National Water Information System data available on the World Wide Web (Water Data for the Nation). http://waterdata.usgs.gov/nwis/. Accessed 2019
Voynova YG, Lebaron KC, Barnes RT, Ullman WJ (2015) In situ response of bay productivity to nutrient loading from a small tributary: the Delaware Bay-Murderkill Estuary tidally-coupled biogeochemical reactor. Estuar Coast Shelf Sci 160:33–48
Walker LM, Montagna PA, Hu X, Wetz MS (2021) Timescales and magnitude of water quality change in three Texas Estuaries induced by passage of Hurricane Harvey. Estuaries Coasts 44:960–971. https://doi.org/10.1007/s12237-020-00846-6
Wang Z, Zhang XX, Lu X, Liu B, Li Y, Long C, Li AM (2014) Abundance and diversity of bacterial nitrifiers and denitrifiers and their functional genes in tannery wastewater treatment plants revealed by high-throughput sequencing. PLoS ONE. https://doi.org/10.1371/journal.pone.0113603
Weber KA, Picardal FW, Roden EE (2001) Microbially catalyzed nitrate-dependent oxidation of biogenic solid-phase Fe(II) compounds. Environ Sci Technol 35:1644–1650
Xu Y, Sun Q, Ye X, Yin X, Li D, Wang L, Wang A, Li Y (2017) Geochemical analysis of sediments from a semi-enclosed bay (Dongshan Bay, southeast China) to determine the anthropogenic impact and source. Chemosphere 174:764–773. https://doi.org/10.1016/j.chemosphere.2017.01.081
Yang WH, Weber KA, Silver WL (2012) Nitrogen loss from soil through anaerobic ammonium oxidation coupled to iron reduction. Nat Geosci 5(8):538–541. https://doi.org/10.1038/Ngeo1530
Zhang M, Krom MD, Lin J, Cheng P, Chen N (2022) Effects of a Storm on the Transformation and export of phosphorus through a subtropical river-turbid Estuary continuum revealed by continuous observation. JGR Biogeosci 127(8):e2022JG006786
Acknowledgements
We gratefully acknowledge support from The Moore Charitable Foundation. We thank Weitao Shuai and Nicole Sherman for their invaluable assistance in field sampling, and the Neuse Riverkeepers for their advice and local insight.
Funding
This work was supported by the Moore Charitable Trust.
Author information
Authors and Affiliations
Contributions
Field work and chemical analyses were conducted by AS. Biological analyses were conducted by SH. Laboratory experiments were conducted by SH and AS. Project was conceived and designed by PJ. All three authors were involved in the data analysis and interpretation. The first draft was written by AS.
Corresponding author
Ethics declarations
Competing interests
None of the authors have any financial interests related to the materials presented here.
Additional information
Responsible Editor: Adam Langley.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sherman, A.E., Huang, S. & Jaffé, P.R. Impacts of storm disturbance and the role of the Feammox process in high nutrient riparian sediments. Biogeochemistry 165, 113–128 (2023). https://doi.org/10.1007/s10533-023-01062-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-023-01062-7