Abstract
Addition of labile carbon (C) inputs to soil can accelerate or slow down the decomposition of soil organic matter (SOM), a phenomenon known as priming effect (PE). However, the magnitude and direction of PE is often difficult to predict, consequently making its relationship with labile C inputs and nutrient availability elusive. To assess this relationship, we added 13C labelled glucose (corresponding to 50% of initial soil microbial biomass C) to two soil types (Vertisol and Acrisol) with different concentrations of available N and from four land use systems (agricultural, pasture, grassland and shrubland). Parallel laboratory incubations i.e. short-term (6 days) and long-term (6 months), were set up to determine the effect of land use and soil type (N availability) on PE. Addition of labelled glucose in solution led to the retardation of SOM mineralization (negative PE) in both soil types and across all land use systems. This is attributed to preferential substrate utilization characterized by the higher mineralization of added glucose. Land use systems and soil types with higher N-availability displayed weaker negative PE, which is in line with the stoichiometric decomposition theory. In conclusion, our study demonstrate that N-availability plays a major role in determining mineralization of labile C inputs, magnitude and direction of PE in the studied dryland soils and land use systems. The fact that 15–27% of the added 13C remained in the soil at the end of the 6 months incubation and PE was negative, indicates that continuous labile C inputs could contribute to C immobilization and stabilization in these semiarid soils. Moreover, 13C glucose remaining in soils after 6 months in semi-natural pastures was comparable to those under natural grassland and shrubland systems especially in Acrisols. This demonstrates that incorporation and maintaining a perennial cover of native pastures has the potential to increase C sequestration in African semi-arid agricultural soils and landscapes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Soils constitute the major reservoir of terrestrial organic carbon (C) accounting for up to 70% of the terrestrial C content (Guenet et al. 2010). The exchange of C between soils and the atmosphere is largely contributed by soil microbial activity (Guenet et al. 2010). Therefore, understanding the mechanisms controlling soil C sequestration and CO2 release to the atmosphere is of significant importance in the context of global climate change. Priming effect (PE) is a strong, short-term change in native soil organic matter (SOM) mineralization induced by different factors such as addition of fresh organic matter, rhizodeposition and fertilization (Kuzyakov 2002). PE is usually measured in the laboratory based on pulse addition of substrates to the soil (Kuzyakov et al. 2000). However, in nature, root exudation (Kuzyakov 2002; Paterson 2003) or plant litter input decomposition and mineralization (Liang et al. 1999) are continuous processes. Previous studies to investigate PE reviewed by Kuzyakov et al. (2000) commonly observed ‘positive priming’ described as the accelerated mineralization of refractory SOM components when stimulated by the addition of labile sources of C. This accelerated SOM turnover might be as a result of increased extracellular enzymes production due to the added substrates, stimulation of microbial activity through fertilization or improvement in soils aeration, moisture and structure (Kuzyakov et al. 2000). However, retardation of SOM mineralization i.e. ‘negative priming’, as a result of new substrate addition may also occur due to the inhibition of microbial activity as a result of changes in the soil environment (Zimmerman et al. 2011). Furthermore, native SOM can also be suppressed due to the divergence of microbial ecological strategy e.g. K-strategists preferentially utilize easily available substrates for their growth than complex soil organic C. This switch of K-strategists consumption from native SOM decomposition to glucose utilization results in negative PE (Blagodatskaya et al. 2007).
Understanding the factors controlling the fluxes of soil organic C is particularly significant for African drylands because: (1) they constitute approximately 60% of the total continental landmass and home to approximately 425 million people (Wei et al. 2019), (2) many tropical soils have been subjected to intense weathering thus characterized by available nutrient deficiency, a major biochemical constraint limiting primary production and SOM decomposition, and (3) prolonged water deficit conditions restricts plant growth, and thus litter inputs that build SOM (Lal 2002). Land degradation across sub-Saharan Africa (SSA) has significantly contributed to the low soil fertility problem especially in the dryland systems (Mganga et al. 2018). Furthermore, due to increased human population influx, African dryland environments are facing increased land cover changes and land use pressure (Mortimore et al. 2005) characterized by conversion of natural ecosystems e.g. grassland, savannas and shrublands to anthropogenic reseeded grass pastures and cropland to satisfy livestock feed and human food demands. In Kenya, around 80% of the land area is affected by degradation (Adams and Watson 2003) and approximately 30–40% of the arid and semi-arid drylands are rapidly being lost with an additional 2% completely denuded (Mganga et al. 2018).
Although fossil-fuel emissions dominate as the source of increasing CO2 concentration in the atmosphere (van Minnen et al. 2009), land use conversions e.g. agricultural expansion have also contributed immensely to the global cumulative atmospheric CO2 increase (Achard et al. 2002). Furthermore, previous studies using soils from Africa (Mganga and Kuzyakov 2018; Razanamalala et al. 2018; Fontaine et al. 2004) demonstrated that addition of different C compounds e.g. glucose and cellulose, accelerated native SOM mineralization. However, these studies only compared different land use systems occurring in a similar soil type in moist and humid environments or in a single land use system and soil type. To our knowledge, studies determining the acceleration or retardation of native SOM mineralization as influenced by: (1) identical land use systems and (2) soil types with different nutrient availability, especially those occurring in African dryland environments, are still lacking. Consequently, fundamental questions about the role of nutrient availability on native SOM turnover especially in African drylands still persist. This is relevant because SOM biodegradation varies widely due to differences in nutrient availability to support microbial communities and processes.
There are two competing hypotheses regarding the impacts of nutrient availability on SOM decomposition i.e. the ‘microbial nitrogen mining hypothesis’ and ‘stoichiometric decomposition hypothesis’ (Craine et al. 2007). The microbial N mining hypothesis i.e. N-limited microorganisms mineralize SOM to obtain the N contained within (Fontaine et al. 2011) is a prominent example used to understand PE mechanisms. This hypothesis explains why CO2 effluxes increases when a soil is supplied with a source of C and energy. When easily available C inputs are added, microorganisms become N-limited and actively degrade organic materials that they would otherwise not degrade to acquire C alone (Garcia-Pausas and Paterson 2011). Consequently, according to the microbial N mining hypothesis, additional SOM mineralization i.e. PE, is negatively correlated to N availability, and positively correlated to C availability (Fontaine et al. 2011). This hypothesis is supported by evidence that increased N availability reduces both native SOM mineralization (Zang et al. 2016) and priming by lower molecular weight organic substances (Garcia-Pausas and Paterson 2011; Fontaine et al. 2011). However, other studies have observed contradictory outcomes (Mganga and Kuzyakov 2018; Tian et al. 2016; Chowdhury et al. 2014). The alternative hypothesis—i.e. the stoichiometric decomposition theory—states that sufficient N supply stimulates microbial growth and SOM decomposition (Chen et al. 2014; Craine et al. 2007). Thus, according to the latter hypothesis, there could be higher positive PE—or less negative PE—in soil with higher N availability. These two hypotheses have been suggested to be competing or contradictory (Chen et al. 2014). We suggest that the N-mining hypothesis does not apply to tropical African soils, which are generally not considered to be N-limited. Therefore, we expect the soils selected for this study to follow the stoichiometric decomposition theory.
To our knowledge, PE and SOM mineralization predictions and the stoichiometric decomposition theory are yet to be tested in soils occurring under different land use systems and with distinct inherent available N (NH4+, NO3−) concentrations, especially in African arid and semi-arid landscapes. For these reasons, we took advantage of identical land use systems (cropland, pasture, grassland and shrubland) occurring in different soil types (Vertisol and Acrisol), and in close proximity to each other, but with very distinct concentration of available nutrients (NH4+, NO3−), in a typical semi-arid dryland in Kenya. The selected land use systems and soil types are a good representation of semi-arid drylands in Kenya. The main objective of this study was to determine the magnitude and direction of PE depending on land use and soil types occurring in Kenyan semi-arid dryland, triggered by labile C input, simulated by 13C labelled glucose addition and try to explain the mechanisms by which they may differ. We anticipate that N-availability will vary more between these two soil types than the land use systems occurring on the same soil type, and thus hypothesize that the magnitude and direction of the PE is influenced more by soil chemical and biological characteristics than common land use systems occurring in a semi-arid environment in Africa.
Materials and methods
Study area
Soils were obtained from the South Eastern Kenya University (SEKU) research farm located in Kitui County (1.421017 °S, 38.024145 °E), a typical semi-arid environment in southeastern Kenya (Schmitt et al. 2019). The area receives an average annual rainfall of around 1000 mm characterized by few intensive storms during the March to May ‘long rains’ and October to December ‘short rains’ with a high annual potential evaporation of 1500–1600 mm, caused by high temperatures and its location near the equator (Lasage et al. 2008). The mean annual temperatures is 20.7 °C (Hayashi 1996). However, the country is experiencing increasing rainfall variability and rising temperatures due to climate change (Schmitt et al. 2019). Soils are well-drained, dark reddish brown to yellowish brown with sandy clay to clay textures supporting different vegetation types and land use systems (Hayashi 1996). For example, Vertisols are churning heavy clay soils formed on deeply weathered parent materials with a high proportion of swelling clays. They occur mainly in tropical and subtropical, semi-arid to subhumid and humid climates supporting a savannah, natural grassland and/or woodland climax vegetation while Acrisols are less weathered and are characterized by lower clay content (Kovda 2020; Strey et al. 2016). Such differences likely affect organic C turnover rates and lead to different responses after changes in land use (Strey et al. 2016).
Soil sampling design, preparation and description of study sites
Soil sampling was done in October, 2017, prior to the start of the short rainy season (October-December), characteristic part of the biannual rainfall regime in semi-arid southern Kenya. Briefly, soils from four (4) land use systems: (1) cropland (intensive use), (2) reseeded pastures (semi-natural), (3) grassland (natural) and (4) shrubland (natural), and two (2) soil types: (1) Vertisol and (2) Acrisol (Table 1) were sampled at the upper 0–10 cm layer using soil cores (5 cm diameter × 10 cm depth). These land use and soil types are typical in semi-arid environments in Africa. Three (3) experimental plots of 50 m × 50 m approximately 1 km apart representing each land use and soil type were sampled. The three sites chosen per land use system and soil type were fair representations of the landscape and region. Soil was sampled from four corners and at the center of each plot, resulting to a total of five positions per sampling. Four soil cores (0–10 cm) were taken per sampling position to obtain representative composite samples. This led to a total of five samples per plot and 15 samples (n) per land use system and soil type. Thereafter, a simple randomization method was used to select three out of the 15 samples for incubation. The randomization was conducted to ensure that the selected samples (n = 3) were obtained from different experimental plots.
Incubation and 13C glucose labelling
Fresh soil (20 g) was placed inside 500 ml glass vessels and pre-incubated for 7 days. Thereafter, 13C labelled glucose, corresponding to 50% of initial soil microbial biomass C, was added as an aqueous solution to the soils, as recommended and used in previous studies (Okolo et al. 2022; Mganga and Kuzyakov 2018; Blagodatskaya et al. 2009). Although glucose is not preferentially utilized by all soil microorganisms, we used it for this study mainly because (1) it is the monomer of most plant-originated organic polymers and most soil microorganisms are capable of metabolizing it and (2) stimulates a large proportion of soil microorganisms (Landi et al. 2006). An equivalent amount of deionized water was added to the control soil samples (without glucose addition, n = 3) and used to observe basal respiration.
After glucose addition, the 500 ml glass vessels were tightly closed with a rubber stopper septum, and a metal screw band, flushed with CO2-free air and incubated at 20 °C for 6 days in a darkened chamber. Soils were maintained at 60% WHC. This is within the range (50–70%) of other previous soil incubation studies to investigate priming effect as affected by land use and soil types in Africa (Okolo et al. 2022; Razanamalala et al. 2018; Mganga and Kuzyakov 2018). During the incubation, CO2 concentrations inside the bottles were measured daily using gas chromatography (Hewlett Packard 6890). For determination of PE, a 20 ml gas sample was taken from the headspace of each glass bottle at the end of the 6 days incubation and injected into a 12 ml evacuated and He-flushed Exetainer. From these samples 13CO2 was analyzed using an isotope ratio mass spectrometer (IRMS) (Thermo Finnigan GasBench II connected to a Thermo Finnigan Delta plus Advantage) (Karhu et al. 2016).
All results represent the cumulative totals at the end of the incubation period. After the gas sampling for 13CO2 determination, the soils were destructively sampled, and extracted for microbial biomass and mineral N concentration (see details below). Another complete set of samples was let to incubate for 6 months in total, and from these samples soil CO2 production was also measured 1 month later to show that the priming effect is a short-term phenomenon, and CO2 production in the samples that received glucose did not remain elevated for longer-term. At 6 months, we measured the 13C content in the soils. This was aimed at determining the amount of added glucose derived C remaining in the two soil types and land use systems in the long-term, and establish how the residual 13C content in the long-term is related to the magnitude of PE and glucose respired in the short-term i.e. first 6 days.
Soil physical and chemical analyses
Air dried soil (oven dried at 40 °C) was used for soil pH, total C and N analyses. Briefly, soil pH was determined in Milli-Q water (ratio of 1:2.5) using a pH meter (WTW InoLab pH Level 1 ba12217e). Aliquots of air-dried soil were ground (mortar and pestle) and used for total C and N analyses. Total organic carbon (TOC) and total nitrogen (TN) were measured using a varioMax CN Elemental Analyzer (Hanau, Germany).
For soil mineral N, about 5 g of fresh sieved soil was extracted with 25 ml 1 M KCl, shaken for 30 min in an orbital shaker (200 rounds per minute) and filtered through SartoriusTM Grade 3-HW folded filters (diameter 150 mm) and stored frozen (− 20 °C) before measuring with an automated flow analyzer Lachat QuikChem 8000 (Zellweger Analytics, Milwaukee, Wisconsin, USA).
Microbial biomass carbon
Microbial biomass C (MBC) was determined at the end of the 6-day incubation period using the chloroform fumigation-extraction (CFE) method (Vance et al. 1987). Briefly, 3 g of fumigated and unfumigated field moist soil samples were extracted with 0.05 M K2SO4 at 1:10 ratio, and k factor of 0.45 (Jörgensen 1996) was used to convert the C released by fumigation into MBC.
Calculating priming effects and statistical analysis
The amount of CO2 (µg CO2-C g soil−1) derived from added glucose (Cglucose) was calculated based on the total cumulative CO2-C amount (Ctotal) and its 13C content (at% sample) at the end of the 6 days incubation period:
where at% glucose is the atom% 13C of the added glucose (2 at%) and at% control is the atom% 13C of control soil-derived CO2. The amount of CO2 originating from the decomposition of soil organic matter (SOM) was calculated as CSOM = Ctotal-Cglucose. PE was then calculated as CSOM-Ccontrol, where (Ccontrol) is the total CO2-C produced by control samples receiving Milli-Q ® water only.
Estimated parameters MBC, cumulative CO2 production from mineralization of native SOM, metabolic quotient, cumulative total CO2-Glucose, were compared between land use and soil types and analyzed using factorial ANOVA (land use and soil types) to test for significant differences. Tukey’s HSD post hoc test was used to separate significant differences between treatments at P < 0.05 significance level (Stat Soft. STATISTICA. V 10.0.) All displayed results represent arithmetic means of 3 replicates ± standard error (SE).
Results
Mineralization of native SOM and microbial biomass C
The effect of land use on SOM mineralization was dependent on soil type. Native SOM mineralization was generally higher in Acrisols compared to Vertisols. However, significant differences were only observed in disturbed cropland and semi-natural pastures. Native SOM mineralization was significantly higher in grassland compared to shrubland in both soil types. Cumulative CO2 produced from native SOM mineralization at the end of the 6-day incubation experiment was highest and lowest in a Acrisol (108.28 ± 2.7 µg CO2–C g−1 soil d.w.) and Vertisol (52.60 ± 2.5 µg CO2–C g−1 soil d.w.) under agricultural production, respectively (Fig. 1). Overall, Vertisols showed greater SOM mineralization in grassland and shrubland (natural systems), and Acrisols displayed higher SOM decomposition in agricultural and pasturelands (agricultural and semi-natural systems).
Microbial biomass C was significantly higher in natural ecosystems compared to disturbed cropland and established pastures. Significant differences in MBC between the soil types were only observed in the natural shrubland ecosystems. A Vertisol shrubland had two-times (1.34 ± 0.09 mg C g−1 soil) MBC compared to Acrisol shrubland (0.65 ± 0.07 mg C g−1). At the end of the 6-day incubation period, the highest and lowest MBC were observed in a Vertisol shrubland (1.34 ± 0.09 mg C g−1 soil) and a Acrisol cropland (0.26 ± 0.07 mg C g−1 soil), respectively (Fig. 2). Glucose addition had a positive (increase), negative (reduction) and neutral (constant) effect on microbial biomass C. However, these observed changes in microbial biomass C were not significant in all land use and soil types.
13C-labelled glucose recovery from CO2 production and bulk soil
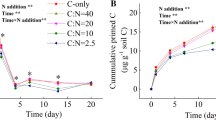
Mineralization of added glucose was consistently and significantly higher in Acrisols compared to Vertisols, across all the land use systems. Significantly higher glucose mineralization was also observed in disturbed agricultural and pasturelands, compared to natural grassland and shrubland. At the end of the 6-day incubation period, 22–43% of the 13C input was mineralized to 13CO2 in all land use systems. The highest and lowest cumulative 13CO2 emissions were observed in an agricultural Acrisol (43.65% ± 1.2) and in a grassland Vertisol (22.35 ± 0.7), respectively (Fig. 3).
Cumulative CO2 from mineralization of 13C labelled glucose during the 6 days incubation depending on soil type and land use. Values are given means ± SE (n = 3). Error bars with different letters are significantly different (P < 0.05). 13CO2 production presented as a percentage of 13C input to compare the effects of land use and soil type on added glucose decomposition
Total CO2 production and rates after 1 month depending on land use and soil type were comparable between the two treatments (i.e. Milli-Q water and glucose treatment) (supplementary material Table 1). This result demonstrates that priming effect is a short-term change in SOM mineralization based on laboratory pulse addition of substrates, and CO2 production in the samples that received glucose did not remain elevated in the longer-term.
At the end of the 6-day incubation period, 50–75% of the 13C labelled glucose remained in the soil and was higher in Vertisols than Acrisols across all the land use systems. Shrubland Vertisol (75.70% ± 1.5) and Vertisol pastureland (52.81% ± 1.3) retained the highest and lowest amount of glucose in soil, respectively (Table 2).
After 6 months, the amount of 13C labelled glucose remaining in soil was significantly lower (P < 0.05) compared to 6-days in both soil types and land use systems. Agricultural Vertisol (14.68% ± 3.8) and shrubland Vertisols (27.30 ± 2.1) retained lowest and the highest amount of glucose in soil, respectively (Table 2).
At the end of the 6 days incubation period, up to 43% of the added glucose was mineralized by microorganisms and released as 13CO2. Across all land use and soil types, the added 13C glucose remaining in soil ranged between 50 and 75%. In total, approximately between 79 and 100% of the initial total 13C was recovered from soil and CO2 efflux.
Priming effect depending on land use and soil type
Generally, addition of glucose resulted to the retardation of SOM mineralization i.e. ‘negative priming’ (Fig. 4). The observed PE was significantly and negatively correlated with available nutrients (NH4+, NO3− and total mineral N) and % C of the control soils before incubation (Figs. 5 and 6). Magnitude of the negative PE varied greatly between soil types and land use systems. Negative PE was significantly stronger (P < 0.05) in Acrisols compared to Vertisols. In Acrisols, reduction in native SOM mineralization after glucose addition was highest in an established pasture (-61.75 ± 1.3 µg CO2–C g−1 soil d.w.) and lowest in a shrubland (− 41.06 ± 3.5 µg CO2–C g−1 soil d.w.) (Fig. 4). However, in Vertisols, the strongest and weakest retardation of native SOM after glucose addition was in a cropland (− 33.95 ± 2.9 µg CO2–C g−1 soil d.w.) and pastureland (− 17.35 ± 3.2 µg CO2–C g−1 soil d.w.) (Fig. 4).
Correlogram of absolute values of negative priming effect and selected characteristics of control soils before incubation. Correlation coefficients are displayed in blue (positive) and red (negative) coloured squares, respectively. Correlations significant at P < 0.05 are marked with an asterisk. The size of the squares is proportional to the correlation coefficients
Discussion
Microbial biomass C and SOM mineralization depending on land use and soil type
Conversion of natural ecosystems for crop and pasture production led to a decline in microbial biomass C. These results compare well with previous studies in the tropics (Singh and Ghoshal 2014; Pabst et al. 2013). High microbial biomass C in natural ecosystems is attributed to continuous and higher organic C inputs in soil. Perennial grassland and shrubland vegetation contribute to an abundant supply of C inputs through root exudation and litter, compared to annual crops, e.g. maize (Kuzyakov and Domanski 2000), which is commonly grown in African drylands. Thus, a continuous supply and accumulation of organic C compounds in natural ecosystems create a conducive microenvironment for microorganisms to thrive, compared to agroecosystems.
Tillage changes the soil physicochemical environment and bare fallow periods characteristic of Kenyan dryland cropping systems accelerate microbial biomass turnover. Similar to our results, Di Lonardo et al. (2019) also reported a reduction in microbial biomass C with increased crop production. Low microbial biomass C in pastures show that intensive pasture production in the studied dryland soils characterized by low fertility, can accelerate microbial turnover. Subsequently, this can affect soil processes and mechanisms linked to nutrient cycling and SOM decomposition (Junior et al. 2018; Galdino et al. 2016). Glucose addition resulted in no significant differences in microbial biomass after 6-day incubation period. This is because the response of microbial biomass to the addition of easily utilizable substrate is short-lived (< 6 days) (Kuzyakov and Bol 2006).
Cropland and pastures under Acrisols with low microbial biomass C produced higher CO2 effluxes from SOM mineralization compared to grassland and shrubland ecosystems with higher microbial biomass C. These results demonstrate that higher biomass does not necessarily lead to higher native SOM mineralization. Thus, low SOM mineralization in natural ecosystems in Acrisols could be indicative of less active soil microbes, decline in microbial turnover and dominance of a microbial community with efficient C use or a low C accessibility. Moreover, it suggests that the quality of C input to be utilized by microbes increases with changes in land use and vegetation cover, leading to the retardation of more recalcitrant native SOM mineralization. Previous studies have also observed higher SOM mineralization in cultivated soils compared to natural ecosystems (Six et al. 2002; Laudicina et al. 2011). Cropland soils had low and comparable microbial biomass C. However, higher CO2 fluxes were observed in Acrisols compared to Vertisols. This difference suggests that the microbial biomass in the former is metabolically more active (Kabiri et al. 2016). Mineralization of SOM in Vertisols was higher in natural ecosystems compared to agroecosystems. A possible explanation to this could be that there a faster and higher microbial turnover in natural compared to agroecosystems in Vertisols.
Soil organic matter mineralization was comparable in soils from similar natural ecosystems. However, higher SOM mineralization was observed in grassland compared to shrubland soils. These differences are attributed to the legacies and footprints of different plant species and functional groups. Plants produce different labile C compounds controlling the quality and quantity of rhizodeposition (root exudates, secretions, detached fine roots and organic compounds from mycorrhizal turnover) in soil (Villarino et al. 2021). Higher accumulation of exudates in grassland soils enabled them to secrete extracellular enzymes responsible for the accelerated SOM mineralization. Similar to our findings, higher CO2 was produced in grassland compared to shrubland soils (Carbone et al. 2008). Belowground grassland productivity is higher than in shrubland, producing more root C inputs available for microbial decomposition (Janssens et al. 2001).
Microbial biomass acts as a C pool and an active driver of C and N mineralization. Thus, primed CO2 can originate from SOM mineralization (real PE) and/or from the increased turnover of microbial biomass (apparent PE). (Kuzyakov et al. 2000). In our study, we did not find a simple linear relationship between negative PE and microbial biomass C. Microbial biomass C content was negatively but not significantly correlated with observed negative PE (Fig. 6). This is indicative that other factors, e.g. soil N availability, had a significant impact on PE, as observed and discussed in other studies (Li et al. 2018; Chen et al. 2014).
Priming effect depending on land use and soil type
Our general results i.e. negative PE across land use and soil type, differ from earlier studies investigating PE in different African soils and land use systems. Positive PE was observed after glucose addition without nutrient fertilization in different land use systems occurring at Mt. Kilimanjaro characterized by Andosols with C/N ratios of 11–16 (Mganga and Kuzyakov 2018). A large-scale case study to investigate the universality of PE also reported positive PE after cellulose addition using agricultural soils from Niger and savannah soils from South Africa and Senegal (Perveen et al. 2019). Moreover, in a recent study, priming effect across different land use systems in semi-arid soils (vertisols and cambisols) of northern Ethiopia with the soil C/N ratio ranging between 9.2 and 102.4 depending on site and land use, was positive in the upper (0–30 cm) depth and negative in subsoils (30–60 cm and 60–90 cm) (Okolo et al. 2022). Observed differences in PE in these studies strongly suggest that several environmental factors such as the amount and stoichiometry of added substrates, inherent organic nutrient availability, microbial community structure play a key role in influencing PE (Di Lonardo et al. 2019). This clearly shows that a concise prediction of the direction (acceleration or retardation of SOM decomposition) and magnitude of PEs in response to organic C additions remains largely uncertain.
In this study, stronger negative PE was observed in Acrisols with higher 13CO2 effluxes, compared to Vertisols that displayed less 13CO2 effluxes. These results support the preferential substrate utilization theory where retardation in SOM mineralization (i.e. negative PE) attributed to addition of a new substrate, e.g. glucose, may occur due to the deviation of microorganisms to the more easily available organic substrate (Kuzyakov et al. 2000; Kuzyakov and Bol 2006). Soil microorganisms switch to utilize added labile C instead of native soil C, as the former is a low-cost energy source (Liu et al. 2017). Similarly, in their study, Blagodatskaya et al. (2007) attributed the observed negative PE to the switch of K-strategists from SOM decomposition to glucose utilization (i.e. preferential substrate utilization). Similarly, negative PE was observed in montane grassland soils from the Peruvian Andes after addition of 200 µg C g−1 soil of xylose, without nutrient addition (Hicks et al. 2019). With the addition of readily available C e.g. glucose and xylose in soils, microorganisms utilize this C source for their growth and energy without the need to mineralize native soil C for nutrient acquisition if nutrients for microorganisms are readily available in the soil (Fontaine et al. 2011).
The magnitude of the negative PE was generally comparable across the different land use types except in a pasture (Vertisol) and shrubland (Acrisol), which had significantly weaker negative PE compared to the other land uses occurring in a similar soil type. Moreover, weaker negative PE observed in nutrient-rich (i.e. higher available N) Vertisols and negative relationship between PE and N-availability (Figs. 4 and 5) suggest that it might be more beneficial for the microorganisms occurring in nutrient-rich soils (i.e. Vertisol) to continue breaking down native SOM (which has a low C:N ratio close to 10, i.e. optimal for stoichiometric growth of microbes) for energy conservation and to acquire nutrients, than changing their metabolic strategy for glucose utilization. This align well with the stoichiometric decomposition’ theory where nutrient-rich conditions are likely to be beneficial for native SOM decomposition (Craine et al. 2007; Chen et al. 2014). This is because mineralization of SOM releases organic compounds (e.g. amino acids, pyruvic acid bases) that can be directly incorporated in their metabolism (Blankinship et al. 2014). This preferential SOM mineralization would also help maintain the K-strategists population by providing the required energy especially during prolonged dry season, characteristic of African dryland environments, when fresh OM is not available. Consequently, these results suggest that the decomposer community in Vertisols occurring in similar dryland environments have evolved and are adapted to decomposition of complex SOM.
13C-labelled glucose recovery depending on land use and soil type
Land use affected the cumulative amount of glucose mineralized. In our study, 22–43% of added 13C-glucose was evolved as 13CO2–C during the 6 days incubation period. This is comparable to other studies in which between 23 and 42% of glucose was evolved as CO2 in less than 7 days (Hoyle et al. 2008; Nguyen and Guckert 2001). Generally, mineralization of added glucose was higher in agroecosystems, which also had lower microbial biomass C, but higher CO2 effluxes from native SOM, compared to natural ecosystems. These results compare well with those of who also observed greater glucose mineralization in cultivated and pasture sites compared to natural ecosystems. Similar to our results, glucose mineralization was much higher in cultivated maize fields compared to natural savannah soils (Mganga and Kuzykov 2018). Our findings demonstrate that soil microbes occurring in the agroecosystems have a preferential utilization for labile C sources. Labile organic C substrates are a more accessible energy source compared to native SOM because of typical limitation of available C to microorganisms in soils. Consequently, these results suggest that even though agricultural and pasture soils in these dryland environments have lost C due to management compared to the natural ecosystems, there is also a great potential for C accumulation if continuous labile C inputs are maintained through improved management e.g. litter incorporation and perennial cover cropping and, if the microbial residues become stabilized in soils. Microbial products formed from low molecular weight organic substrates e.g. glucose, are the prerequisite for C stabilization in soils (Gunina and Kuzyakov 2015).
Similar to land use, mineralization of added glucose varied between soil types. This is mainly attributed to inherent differences in mineral N-availability (NO3− and NH4+). Decomposition of glucose was consistently higher in Acrisols, with lower concentration of NO3− and NH4+ than Vertisols, across all land use systems. These results compare well with those of Conde et al. (2005) who also observed a retardation of glucose mineralization with increased N availability in soils during a 7-day incubation period. However, results from other studies have also demonstrated an increase (Creamer et al. 2016; Conde et al. 2005) and neutral effect (Dimassi et al. 2014) in the decomposition of low molecular weight compounds with increased N-availability. Our results strongly suggest that the direction and magnitude of mineralization of easily available C substrates in similar semi-arid landscapes is mainly influence by nutrient (N) availability than land use.
The amount of 13C derived from the added glucose, but transformed by soil microbes into more stable forms, and thus remaining in soil after 6 months incubation period (% of input) were comparable between the soil types (Vertisols 14–27% and Acrisols 19–26%, respectively) and were in the order natural (grassland and shrubland) (23–27%) > semi-natural (pasture) (17–26%) > agricultural (15–19%) systems, respectively. Higher total % N in natural and semi-natural soils compared to agricultural systems could have fueled microbial growth with organic C addition. Moreover, increased N content has a positive impact on the transformation of residue C e.g., microbial residues, to form more stable SOM (Moran et al. 2005). This was confirmed in our study where we observed lower CO2 produced from mineralization of added glucose in natural compared to semi-natural and agricultural soils. This is because microbial CUE is usually higher at conditions of higher N content, and large CUE indicates a higher potential for the C to become stabilized in soil as microbial residues (Luo et al. 2020).
Conclusions
Higher glucose mineralization corresponded to the retardation of SOM mineralization (negative PE) across the studied land use systems and soil types occurring in a typical semi-arid environment in Africa. This pattern confirms to the preferential substrate utilization theory as a factor contributing to negative PE. Magnitude and direction of the observed negative PE was greatly influenced by N availability and SOM. Higher utilization of labile C inputs for respiration and corresponding retardation in native SOM decomposition in Acrisols demonstrate their greater potential for C accumulation, if the microbial biomass residues become stabilized in soils. Higher glucose remaining in Acrisols under agricultural and pasture systems demonstrate greater potential for C accumulation. Thus, this study shows that even though agricultural and pasture soils in these dryland environments have lost C due to management compared to the natural ecosystems, there is also a great potential for C accumulation if continuous labile C inputs are maintained through improved land and agricultural management e.g., litter incorporation and perennial cover cropping.
Data availability
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
Achard F, Eva HD, Stibig HJ, Mayaux P, Gallego J, Richards T, Malingreau J-P (2002) Determination of deforestation rates of the world’s humid tropical forests. Science 297:999–1002. https://doi.org/10.1126/science.1070656
Adams WM, Watson EE (2003) Soil erosion, indigenous irrigation and environmental sustainability, Marakwet, Kenya. Land Degrad Dev 14:109–122. https://doi.org/10.1002/ldr.528
Blagodatskaya EV, Blagodatsky SA, Anderson TH, Kuyakov Y (2007) Priming effect in Chernozem induced by glucose and N in relation to microbial growth strategies. Appl Soil Ecol 37:95–105. https://doi.org/10.1016/j.apsoil.2007.05.002
Blagodatskaya EV, Blagodatsky SA, Anderson TH, Kuzyakov Y (2009) Contrasting effects of glucose, living roots and maize straw on microbial growth kinetics and substrate availability in soil. Eur J Soil Sci 60:186–197. https://doi.org/10.1111/j.1365-2389.2008.01103.x
Blankinship JC, Becerra CA, Schaeffer SM, Schimel JP (2014) Separating cellular metabolism from exoenzyme activity in soil organic matter decomposition. Soil Biol Biochem 71:68–75. https://doi.org/10.1016/j.soilbio.2014.01.010
Carbone MS, Winston GC, Trumbore SE (2008) Soil respiration in perennial grass and shrub ecosystems: linking environmental controls with plant and microbial sources on seasonal and diel times. J Geophys Res 113:G02022. https://doi.org/10.1029/2007JG000611
Chen R, Senbayram M, Blagodatsky S, Myachina O, Dittert K, Lin X, Blagodatskaya E, Kuzyakov Y (2014) Soil C and N availability determine the priming effect: microbial N mining and stoichiometric decomposition theories. Glob Change Biol 20:2356–2367. https://doi.org/10.1111/gcb.12475
Chowdhury S, Farrell M, Bolan N (2014) Priming of soil organic carbon by malic acid addition is differentially affected by nutrient availability. Soil Biol Biochem 77:158–169. https://doi.org/10.1016/j.soilbio.2014.06.027
Conde E, Cardenas M, Ponce-Mendoza A, Luna-Guido ML, Cruz-Mondragón C, Dendooven L (2005) Impacts of inorganic nitrogen application on mineralization of 14C-labelled maize and glucose, and on priming effect in saline alkaline soil. Soil Biol Biochem 37:681–691. https://doi.org/10.1016/j.soilbio.2004.08.026
Craine JM, Morrow C, Fierer NO (2007) Microbial nitrogen limitation increases decomposition. Ecology 88:2105–2113. https://doi.org/10.1890/06-1847.1
Creamer CA, Jones DL, Baldock JA, Rui Y, Murphy DV, Hoyle FC, Farrel M (2016) Is the fate of glucose-derived carbon more strongly driven by nutrient availability, soil texture or microbial biomass size? Soil Biol Biochem 103:201–212. https://doi.org/10.1016/j.soilbio.2016.08.025
Di Lonardo DP, de Boer W, Zweers H, van der Wal A (2019) Effect of the amount of organic trigger compounds, nitrogen and soil microbial biomass on the magnitude of priming of soil organic matter. PLoS ONE 14(5):e0216730. https://doi.org/10.1371/journal.pone.0216730
Dimassi B, Mary B, Fontaine S, Perveen N, Revaillot S, Cohan JP (2014) Effect of nutrients availability and long-term tillage on priming effect and soil C mineralization. Soil Biol Biochem 78:332–339. https://doi.org/10.1016/j.soilbio.2014.07.016
Fontaine S, Bardoux G, Abbadie L, Mariotti A (2004) Carbon input to soil may decrease soil carbon content. Ecol Letters 7:314–320. https://doi.org/10.1111/j.1461-0248.2004.00579.x
Fontaine S, Henault C, Aamor A, Bdioui N, Bloor JMG, Maire V, Mary B, Revaillot S, Maron PA (2011) Fungi mediate long term sequestration of carbon and nitrogen in soil through their priming effect. Soil Biol Biochem 43:86–96. https://doi.org/10.1016/j.soilbio.2010.09.017
Galdino S, Sano EE, Andrade RG, Grego CR, Nogueira SF, Bragantini C, Flosi AHG (2016) Large-scale modeling of soil erosion with RUSLE for conservationist planning of degraded cultivated Brazilian pastures. Land Degrad Dev 27:773–784. https://doi.org/10.1002/ldr.2414
Garcia-Pausas J, Paterson E (2011) Microbial community abundance and structure are determinants of soil organic matter mineralisation in the presence of labile carbon. Soil Biol Biochem 43:1705–1713. https://doi.org/10.1016/j.soilbio.2011.04.016
Guenet B, Leloup J, Raynaud X, Bardoux G, Abbadie L (2010) Negative priming effect on mineralization in a soil free of vegetation for 80 years. Eur J Soil Sci 61:384–391. https://doi.org/10.1111/j.1365-2389.2010.01234.x
Gunina A, Kuzyakov Y (2015) Sugars in soil and sweets for microorganisms: review of origin, content, composition and fate. Soil Biol Biochem 90:87–100. https://doi.org/10.1016/j.soilbio.2015.07.021
Hayashi I (1996) Five years experiment on vegetation recovery of drought deciduous woodland in Kitui, Kenya. J Arid Environ 34:351–361. https://doi.org/10.1006/jare.1996.0115
Hicks LC, Meir P, Nottingham AT, Reay DS et al (2019) Carbon and nitrogen inputs differentially affect priming of soil organic matter in tropical lowland and montane soils. Soil Biol Biochem 129:212–222. https://doi.org/10.1016/j.soilbio.2018.10.015
Hoyle FC, Murphy DV, Brookes PC (2008) Microbial response to the addition of glucose in low-fertility soils. Biol Fertil Soils 44:571–579. https://doi.org/10.1007/s00374-007-0237-3
Janssens IA, Lankreijer H, Matteucci G, Kowalski AS et al (2001) Productivity overshadows temperature in determining soil and ecosystem respiration across European forests. Glob Change Biol 7:269–278. https://doi.org/10.1046/j.1365-2486.2001.00412.x
Jörgensen RG (1996) The fumigation-extraction method to estimate soil microbial biomass: calibration of the kEC value. Soil Biol Biochem 28:25–31. https://doi.org/10.1016/0038-0717(95)00102-6
Junior FMC, Carneiro RFV, Rocha SMB, Nunes LAPL et al (2018) The impact of pasture systems on soil microbial biomass and community-level physiological profiles. Land Degrad Dev 29:284–291. https://doi.org/10.1002/ldr.2565
Kabiri V, Raiesi F, Ghazavi MA (2016) Tillage effects on soil microbial biomass, SOM mineralization and enzyme activity in a semi-arid calcixerepts. Agric Ecosyst Environ 232:73–84. https://doi.org/10.1016/j.agee.2016.07.022
Karhu K, Hilasvuori E, Fritze H, Biasi C, Nykänen H, Liski J, Vanhala P, Heinonsalo J, Pumpanen J (2016) Priming effect increased with depth in a boreal forest soil. Soil Biol Biochem 99:104–107. https://doi.org/10.1016/j.soilbio.2016.05.001
Kovda I (2020) Vertisols: Extreme features and extreme environment. Geoderma Reg 22:e00312. https://doi.org/10.1016/j.geodrs.2020.e00312
Kuzyakov Y (2002) Review: factors affecting rhizosphere priming effects. J Plant Nutr Soil Sc 165:382–396. https://doi.org/10.1002/1522-2624(200208)165:4%3c382::AID-JPLN382%3e3.0.CO;2-%23
Kuzyakov Y, Bol R (2006) Sources and mechanisms of priming effect induced in two grassland soils amended with slurry and sugar. Soil Biol Biochem 38:747–758. https://doi.org/10.1016/j.soilbio.2005.06.025
Kuzyakov Y, Domanski G (2000) Carbon input by plants into the soil. J Plant Nutr Soil Sc 163:421–431. https://doi.org/10.1002/1522-2624(200008)163:4%3c421::AID-JPLN421%3e3.0.CO;2-R
Kuzyakov Y, Friedel JK, Stahr K (2000) Review of mechanisms and quantification of priming effects. Soil Biol Biochem 32:1485–1498. https://doi.org/10.1016/S0038-0717(00)00084-5
Lal R (2002) Carbon sequestration in dryland ecosystems of west Asia and north Africa. Land Degrad Dev 13:45–59. https://doi.org/10.1002/ldr.477
Landi L, Valori F, Ascher J, Renella G, Falchini L, Nannipieri P (2006) Root exudate effects on the bacterial communities, CO2 evolution, nitrogen transformations and ATP content of rhizosphere and bulk soils. Soil Biol Biochem 38:509–516. https://doi.org/10.1016/j.soilbio.2005.05.021
Lasage R, Aerts J, Mutiso GCM, de Vries A (2008) Potential for community based adaptation to droughts: sand dams in Kitui, Kenya. Phys Chem Earth 33:67–73. https://doi.org/10.1016/j.pce.2007.04.009
Laudicina VA, Badalucco L, Palazzolo E (2011) Effects of compost input and tillage intensity on soil microbial biomass and activity under Mediterranean conditions. Biol Fertil Soils 47:63–70. https://doi.org/10.1007/s00374-010-0502-8
Li L-J, Zhu-Barker X, Ye R, Doane TA, Horwath WR (2018) Soil microbial biomass size and soil carbon influence the priming effect from carbon inputs depending on nitrogen availability. Soil Biol Biochem 119:41–49. https://doi.org/10.1016/j.soilbio.2018.01.003
Liang BC, Gregorich EG, MacKenzie AF (1999) Short-term mineralization of maize residues in soils as determined by carbon-13 natural abundance. Plant Soil 208:227–232. https://doi.org/10.1023/A:1004543231568
Liu XJ, Sun J, Mau RL, Finley BK et al (2017) Labile carbon input determines the direction and magnitude of the priming effect. Appl Soil Ecol 109:7–13. https://doi.org/10.1016/j.apsoil.2016.10.002
Luo R, Kuzyakov Y, Liu D, Fan J, Luo J, Lindsey S, He J-S, Ding W (2020) Nutrient addition reduces carbon sequestration in a Tibetan grassland soil: disentangling microbial and physical controls. Soil Biol Biochem 144:107764. https://doi.org/10.1016/j.soilbio.2020.107764
Mganga KZ, Kuzyakov Y (2018) Land use and fertilisation affect priming in tropical andosols. Eur J Soil Biol 87:9–16. https://doi.org/10.1016/j.ejsobi.2018.04.001
Mganga KZ, Nyariki DM, Musimba NKR, Amwata DA (2018) Determinants and rates of land degradation: application of stationary time-series model to data from semi-arid environment in Kenya. J Arid Land 10:1–11. https://doi.org/10.1007/s40333-017-0036-0
Moran KK, Six J, Horwath WR, van Kessel C (2005) Role of mineral-Nitrogen in residue decomposition and stable soil organic matter formation. Soil Sci Soc Am J 69:1730–1736. https://doi.org/10.2136/sssaj2004.0301
Mortimore M, Ba M, Mahamane A, Rostom RS, del Pozo PA, Turner B (2005) Changing systems and changing landscapes: measuring and interpreting land use transformation in African drylands. Geogr Tidsskr 105:101–118. https://doi.org/10.1080/00167223.2005.10649530
Nguyen C, Guckert A (2001) Short-term utilization of 14C-[U] glucose by soil microorganisms in relation to carbon availability. Soil Biol Biochem 33:53–60. https://doi.org/10.1016/S0038-0717(00)00114-0
Okolo CC, Bore E, Gebresamuel G, Zenebe A, Haile M, Nwite JN, Dippold MA (2022) Priming effect in semi-arid soils of northern Ethiopia under different land use types. Biogeochemistry 158:383–403. https://doi.org/10.1007/s10533-022-00905-z
Pabst H, Kühnel A, Kuzyakov Y (2013) Effect of land use and elevation on microbial biomass and water extractable carbon in soils of Mt Kilimanjaro ecosystems. Appl Soil Ecol 67:10–19. https://doi.org/10.1016/j.apsoil.2013.02.006
Paterson E (2003) Importance of rhizodeposition in the coupling of plant and microbial productivity. Eur J Soil Sci 54:741–750. https://doi.org/10.1046/j.1351-0754.2003.0557.x
Perveen N, Barot S, Maire V, Cotrufo MF, Shahzad T, Blagodatskaya E et al (2019) Universality of priming effect: an analysis using thirty five soils with contrasted properties sampled from five continents. Soil Biol Biochem 134:162–171. https://doi.org/10.1016/j.soilbio.2019.03.027
Razanamalala K, Fanomezana RA, Razafimbelo T, Chevallier T, Trap J, Blanchart E, Bernard L (2018) The priming effect generated by stoichiometric decomposition and nutrient mining in cultivated tropical soils: Actors and drivers. Appl Soil Ecol 126:21–33. https://doi.org/10.1016/j.apsoil.2018.02.008
Schmitt CB, Kisangau D, Matheka KW (2019) Tree diversity in a human modified riparian forest landscape in semi-arid Kenya. For Ecol Manag 433:645–655. https://doi.org/10.1016/j.foreco.2018.11.030
Singh MK, Ghoshal N (2014) Variation in soil microbial biomass in the dry tropics: impact of land-use change. Soil Res 52:299–306. https://doi.org/10.1071/SR13265
Six J, Conant T, Paul A, Paustian K (2002) Stabilization mechanisms of soil organic matter: implications for C-saturation of soils. Plant Soil 241:155–176. https://doi.org/10.1023/A:1016125726789
Strey S, Boy J, Strey R, Weber O, Guggenberger G (2016) Response of soil organic carbon to land-use change in central Brazil: a large-scale comparison of Ferralsols and Acrisols. Plant Soil 408:327–342. https://doi.org/10.1007/s11104-016-2901-6
Tian Q, Yang X, Wang X, Liao C, Li Q, Wang M, Wu Y, Liu F (2016) Microbial community mediated response of organic carbon mineralization to labile carbon and nitrogen addition in topsoil and subsoil. Biogeochemistry 128:125–139. https://doi.org/10.1007/s10533-016-0198-4
Van Minnen JG, Goldewijk KK, Stehfest E, Eickhout B, van Drecht G, Leemans R (2009) The importance of three centuries of land-use change for the global and regional terrestrial carbon cycle. Clim Chang 97:123–144. https://doi.org/10.1007/s10584-009-9596-0
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass C. Soil Biol Biochem 19:703–707. https://doi.org/10.1016/0038-0717(87)90052-6
Villarino S, Pinto P, Jackson RB, Piñeiro G (2021) Plant rhizodeposition: a key factor for soil organic matter formation in stable fractions. Sci Adv 7:eabd3176. https://doi.org/10.1126/sciadv.abd3176
Wei F, Wang S, Fu B, Wang L, Liu YY, Li Y (2019) African dryland ecosystem changes controlled by soil water. Land Degrad Dev 30:1564–1573. https://doi.org/10.1002/ldr.3342
Zang H, Wang J, Kuzyakov Y (2016) N fertilization decreases soil organic matter decomposition in the rhizosphere. Appl Soil Ecol 108:47–53. https://doi.org/10.1016/j.apsoil.2016.07.021
Zimmerman AR, Gao B, Ahn M-Y (2011) Positive and negative carbon mineralization priming effects among a variety of biochar-amended soils. Soil Biol Biochem 43:1169–1179. https://doi.org/10.1016/j.soilbio.2011.02.005
Acknowledgements
This research was supported by funds from the Academy of Finland (Grant number 316401) and Helsinki Institute of Life Science (HiLIFE) Fellow funding for K.K. We thank Marjut Wallner for help with C and N analysis.
Funding
Open Access funding provided by University of Helsinki including Helsinki University Central Hospital. This research was supported by funds from the Academy of Finland (Grant number 316401) and Helsinki Institute of Life Science (HiLIFE) Fellow funding for Kristiina Karhu.
Author information
Authors and Affiliations
Contributions
All the authors contributed to the study conception and design. Material preparation, data collection were performed by KZM, JLR and SK, sample preparation and analysis were performed by KZM, CB and KK. The first draft of the manuscript was written by KZM and all authors commented and approved on previous versions of the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Responsible Editor: Kate Lajtha.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mganga, K.Z., Rolando, J.L., Kalu, S. et al. Priming effect depending on land use and soil types in a typical semi-arid landscape in Kenya. Biogeochemistry 163, 49–63 (2023). https://doi.org/10.1007/s10533-023-01016-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-023-01016-z