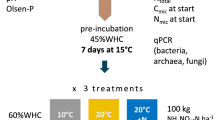
Abstract
Climate change currently manifests in upward and northward shifting treelines, which encompasses changes to the carbon (C) and nitrogen (N) composition of organic inputs to soils. Whether these changed inputs will increase or decrease microbial mineralisation of native soil organic matter remains unknown, making it difficult to estimate how treeline shifts will affect the C balance. Aiming to improve mechanistic understanding of C cycling in regions experiencing treeline shifts, we quantified priming effects in soils of high altitudes (Peruvian Andes) and high latitudes (subarctic Sweden), differentiating landcover types (boreal forest, tropical forest, tundra heath, Puna grassland) and soil horizons (organic, mineral). In a controlled laboratory incubation, soils were amended with substrates of different C:N, composed of an organic C source at a constant ratio of 30% substrate-C to microbial biomass C, combined with different levels of a nutrient solution neutral in pH. Substrate additions elicited both positive and negative priming effects in both ecosystems, independent from substrate C:N. Positive priming prevailed above the treeline in high altitudes and in mineral soils in high latitudes, where consequently climate change-induced treeline shifts and deeper rooting plants may enhance SOM-mineralisation and soil C emissions. However, such C loss may be compensated by negative priming, which dominated in the other soil types and was of larger magnitude than positive priming. In line with other studies, these results indicate a consistent mechanism linking decreased SOM-mineralisation (negative priming) to increased microbial substrate utilisation, suggesting preferential substrate use as a potential tool to support soil C storage.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Climate change can alter plant community composition and species distributions, which can change entire landscapes (Harsch et al. 2009; van der Putten et al. 2010; Körner and Paulsen 2014; Feeley et al. 2020). It also changes the finely tuned interactions between plants, soils and microbes, and particularly how carbon (C) and nitrogen (N) are distributed between the atmosphere and soils. Ecosystems in high altitudes and high latitudes share two features which are of particular importance in the context of climate change: their soils have large C stocks (Zimmermann et al. 2010; Saatchi et al. 2011; Rolando et al. 2017; Yang et al. 2018) and these biomes are predicted to experience greater than average increases in temperature (Wookey et al. 2009; Classen et al. 2015; Wang et al. 2016), which has raised concerns about their function as global C sinks (Keuper et al. 2020; Nottingham et al. 2020). The direct abiotic impacts of climate change are increasingly well studied in these vulnerable ecosystems. However, the indirect biotic effects, like large-scale species shifts, and the related feedback on soil elemental cycling, remain widely uncertain.
Treeline shifts can have contrasting effects on the C cycle and terrestrial C stocks. Increasing amounts of above- and belowground biomass and greater recalcitrance of litter from different species can increase carbon dioxide (CO2) uptake and the potential for new soil organic matter (SOM) formation (Lange et al. 2015; Rolando et al. 2017; Sullivan et al. 2020). Yet, greater C and nutrient inputs do not always result in greater C storage, as plant litter inputs and root exudation can also enhance SOM mineralization by microbes. This phenomenon of altered SOM mineralisation in response to fresh organic inputs is known as ‘priming effect’ (Löhnis 1926; Bingemann et al. 1953; but see Kuzyakov et al. 2000). A positive priming effect refers to a situation where SOM mineralisation is enhanced following labile inputs. Accordingly, a negative priming effect describes reduced rates of SOM-mineralisation after substrate addition. Positive priming has the potential to outweigh the C capture in biomass through mobilisation of the belowground C stocks, a major concern particularly for arctic ecosystems under climate change (Hartley et al. 2012; Wild et al. 2014; Parker et al. 2015, 2021; Keuper et al. 2020). Priming effects also concern ecosystem modellers, as they may undermine the suitability of applying first order kinetics to decomposition processes (Perveen et al. 2014).
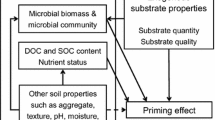
It is theorized that priming effects result from the interplay of supply and demand of energy, usually C compounds, and nutrients that are exchanged between microbes and plants (Kuzyakov and Cheng 2001; Jones et al. 2009; Dijkstra et al. 2013; Murphy et al. 2015; Qiao et al. 2016; Wang et al. 2016; Averill and Waring 2017; Soong et al. 2018). The mechanistic basis of priming effects remains however controversial. According to the microbial N-mining hypothesis, the addition of labile C to soils with low N availability (high C:N) increases the microbial demand for N, stimulating the mineralisation of SOM (positive priming) as microbes strive to meet their nutritional needs by releasing more N from soil (Schimel and Weintraub 2003; Craine et al. 2007; Chen et al. 2014). On the other hand, the prediction according to preferential substrate use is that the addition of labile C with sufficiency of nutrients decreases the mineralisation of SOM (negative priming), as microbes shift from mineralizing SOM to using substrate-C as their primary nutrient source instead (Cheng 1999; Blagodatskaya et al. 2011; Wang et al. 2015).
Compared to temperate grasslands and forests, organic inputs in undisturbed high altitudinal and high latitudinal ecosystems are considerably lower, with seasonal changes increasing C allocation to either above or belowground plant parts according to summer/winter or dry/wet climate transitions (Kaiser et al. 2010; Girardin et al. 2016). In both Andean and subarctic ecosystems, N is considered as a limiting factor and determinant for plant and microbial performance (Weintraub and Schimel 2005; Buckeridge et al. 2010; Nottingham et al. 2012; Fisher et al. 2013). In this laboratory incubation study, we amended soils from above and below the treeline in high altitudes and high latitudes with low rates of substrates of varying C:N to develop mechanistic understanding of how priming effects are controlled in these contrasting N-limited ecosystems. We investigated the divergence of predicted priming effects according to either N-mining (positive priming) or preferential substrate use (negative priming), considering how the C:N of organic inputs interacts with the inherent C and N differences in soil and microbial biomass of the studied ecosystems.
We hypothesised that (H1) the soils above the treeline (Puna grasslands in the Andes and tundra heath in the Arctic) would be more susceptible to positive priming than their forest counterparts, due to stronger microbial N-demand. Positive priming would lead to N-mining, which should be more pronounced when C-rich, N-poor substrates are added and decrease with increasing substrate-N content. On the other hand (H2), we hypothesised that when microbes switch their energy (carbon) and nutrient (nitrogen) acquisition from soil to substrate, this preferential substrate use causes negative priming. We expected this particularly in cases where the C:N of the added substrate was close to the C:N of the microbial biomass of the receiving soil, as processes of microbial anabolism would have the least stoichiometric constraints.
Material and methods
Study sites
Soils were collected in 2016 in the high altitudes of the Peruvian Andes in Manú National Park in the department of Cusco at an average elevation of 3300 m (13° 07′ S 71° 36′ W), and in the high latitudes of the boreal subarctic near the Abisko Scientific Research Station, 250 km north of the Arctic Circle in Northern Sweden (68° 21′ N 18° 49′ E).
The study area in the Peruvian Andes is situated at the high end of the Kosñipata transect on the Eastern side of the Andes, on the Western-facing hill side of the Paucartambo river valley. The study area comprises a montane tropical forest with a short transition zone leading into Puna grassland. The forest is a high Andean tropical mountain forest dominated by Weinmannia microphylla (Kunth), Polylepis pauta (Hieron.) and Gynoxys induta (Cuatrec.). The adjacent Puna grasslands are mainly composed of the genera Festuca, Hypericum and Carex. The climate is characterised by a rainy season from October to April, but in the forest and at the treeline cloud cover can be dense and humidity high throughout the year. The mean temperature is around 13 °C at the treeline, but can reach up to 25 °C in October and cool down to 3–6 °C in the Puna (UNEP World Conservation Monitoring Centre 2017). The soils referred to as “Andean soils” in this study are derived from volcanic material with mostly low base status. Because of diverse topography, slope and exposure, and due to varying history of erosion and landslides, these soils represent a variety of soil types. The forest soils are mostly Cambisols with a large fraction of organic matter in the upper soil horizon. The Puna grassland soils are shallower and mostly Andosols, where the sub soil contains notable quantities of amorphous clay (FAO Soil map of the world 1971; Wilcox et al. 1988; FAO World reference base for soil resources 2015).
The study region in the Swedish subarctic is located near Abisko, south of the lake Torneträsk. The treeline transition along the elevational gradient has a Northeast—Southwest orientation. The studied treeline forms the upper end of a fragmented birch forest, which fades into alpine tundra. The dominant canopy-forming species of the studied birch forests is Betula pubescens (Ehrh.), while at some sites Betula nana (L.), Salix glauca (L.) and Juniper sp. are also present. The forest understorey is mostly composed of ericaceous plants such as Empetrum nigrum (L.), and several species of Vaccinium. The plant species composition of the upland heath lands is similar to the forest understorey, mainly composed of dwarf shrubs and cryptogams. In contrast to the Andean uplands, true grasses are widely absent, but species of the genera Lycopodium and Equisetum are commonly present at low abundance. There is regularly snow on the ground until late May and while average temperatures may be a little over 10 °C in July, by mid-August the average temperature is already declining rapidly with frosts likely by early September. In winter, temperatures can drop down to—34 °C. Precipitation averages 15 mm per month during the year, with July and August being wetter (60 mm/month; Abisko Scientific Research Station). Bedrock is formed by salic igneous rocks and quartic and phyllitic hard schists (Sundqvist et al. 2011). The subarctic soils referred to as “Boreal soils” in this study are permafrost-free and mostly Podsols and Cambisols with thin organic rich topsoils and sandy mineral soils from the B-horizon (FAO World reference base for soil resources 2015).
Soil sampling
In both countries, the sampling area covered approximately 450 km2, in which six individual sampling locations were identified with 2–8 km between them. At each sampling location, a 30 m transect was marked orthogonally to the treeline, positioning the transition zone between the timberline and the tree species line at its middle (Berdanier 2010). Hence, for each transect, one end point was inside the forest below the treeline and the other one in the corresponding upland above the treeline (Puna grassland or tundra heath). At each end point of each transect, a plot of 15 × 15 m (225 m2) was established, 24 plots in total (6 × Andean mountain forest, 6 × Andean Puna grassland, 6 × boreal birch forest, 6 × boreal tundra heath).
Within each plot, soils were collected at five sampling points spaced out like the five dots on a dice (⁙). At each of these, approximately five litres of soil were sampled separately from organic and mineral soil horizons. For the organic soils, the litter layer in the forests was removed before sampling. In the Puna grasslands and tundra heath, dense root mats were not included in the samples and mineral soils were sampled without large rocks. The five samples of each plot were combined into one composite sample, which was homogenized by hand and approximately 20 L were then sealed in plastic bags and stored at 4 °C until the experiment was run in spring 2017. The total number of samples was 48 (4 land cover types × 2 horizons × 6 field replicates). Within each plot, additional intact soil cores (h = 15 cm, d = 5 cm) were taken, one each from the organic and mineral soil horizons, to determine bulk density.
For this experiment, we classified eight soil types representing the treeline ecotone based on the soil origin in terms of geographic region (Andean, Boreal), native current land cover (tropical mountain or boreal Forest, Puna grassland, Tundra heath) and soil horizon (Organic, Mineral). We follow the same labelling throughout the manuscript, defining the soils by these three characteristics as: Andean Forest Organic (AFO), Andean Forest Mineral (AFM), Andean Puna organic (APO), Andean Puna Mineral (APM), Boreal Forest Organic (BFO), Boreal Forest Mineral (BFM), Boreal Tundra Organic (BTO) and Boreal Tundra Mineral (BTM).
Pre-incubation analysis
For each composite sample, we determined (in order of increasing analytical precision): soil texture, bulk density (BD), maximum water holding capacity (max WHC), soil pH, total C and N contents and extractable N (Table 1). Soil texture was first assessed in the field following standard protocols (VD LUFA I, D 2.1 1997) and then specified in the lab following standard practice (Davis and Bennett 1927; Sitton and Story 2006). To calculate field bulk density (BD), the mass and volume of rocks and roots were determined and subtracted from the soil mass and volume from 15 cm soil cores (d = 5 cm), which were taken in each landcover type and for each soil horizon individually. Soils were sieved to 2 mm. Dry matter and water content were determined by drying soil samples at 105 °C until constant weight (Schlichting and Blume 1967). Maximum WHC was calculated as the difference in weight of soil at field capacity (saturated soil after draining) and dry soil. Soil pH was measured using a Hanna HI-111 pH/ORP meter (Hanna Instruments, Woonsocket, Rhode Island, USA) according to Emmett et al. (2008). For each sample, 10 g of field moist soil was mixed with 25 ml deionised water, stirred and allowed to settle overnight, before the pH was recorded. Total soil C and N concentrations were analysed on 5 g oven-dried (105 °C) sub-samples, which were ground and analysed via combustion and thermal conductivity detection (Elemental analyser Vario EL, Elementar, Langenselbold, Hessen, Germany). Sub-samples of 5 g soil each were extracted with 0.5 M potassium sulfate (K2SO4) for analysis of mineral N in form of extractable ammonium (NH4+) and nitrate (NO3−) and extracts were colorimetrically analysed (Bran Luebbe AA3 AutoAnalyzer Bran and Luebbe, Northampton, East Midlands, UK).
Microbial biomass C and N were analysed using the direct extraction method (Tate et al. 1988; Gregorich et al. 1990; Fierer and Schimel 2003): A K2SO4-salt solution was used as extractant and liquid ethanol-free chloroform (CHCl3 stabilised with amylene) directly added to release C and N from microbial cells. All soils were analysed in duplicate, where one sample was extracted with salt solution only (5 g fresh weight soil + 25 ml 0.5 M K2SO4, pH adjusted to 6.8–7/w NaOH) and the other sample was additionally treated with liquid CHCl3 (5 g fresh weight soil + 25 ml 0.5 M K2SO4, pH adjusted to 6.8–7/w NaOH + 0.5 ml CHCl3). Extractable microbial C and N of all samples was analysed using a TOC/TN analyser (5000A, Shimadzu, Milton Keynes, Buckinghamshire, UK) and biomass C and N were calculated by subtracting the C and N contents of the salt-extracted samples from the element contents of the chloroformed samples. Given the heterogeneity of soil types studied, we present the microbial biomass C and N data as the actual values that were measured without applying general correction factors, as recommended in Halbritter et al. (2020), protocol 2.2.1 (Schmidt, I.K., Reinsch, S., Christiansen, C.T.).
Preparing the substrate solutions
All substrate additions included 10% isotopically enriched glucose (Cambridge Isotope Laboratories, Tewksbury, Massachusetts, USA) as a C source. To avoid substantial increases in microbial biomass and changes in microbial community composition, as well as an “apparent priming” effect, all substrate-C additions were proportional to 30% of respective soil microbial biomass C contents (Blagodatskaya and Kuzyakov 2008; Blagodatsky et al. 2010; Blagodatskaya et al. 2011). Substrate-C additions were calculated based on the average microbial biomass C of organic and mineral soil horizons separately. Four substrate treatments were prepared: One treatment was glucose only (“glu”) and three additional substrate treatments were made with combined glucose and N additions. For each soil type, the same amount of glucose was dissolved in different concentrations of Hoagland’s No.2 solution (H2395, Sigma Aldrich, Burlington, Massachusetts, USA; supplementary material 1) to obtain the final C:N 71:1 (“glu + N”), 17:1 (“glu + NN”) and 7:1 (“glu + NNN”), where C:N 7:1 has the highest N content and 71:1 the lowest N content (Table 2). The final C:N of the substrates mimicked natural resources, such as microbial biomass, SOM and leaf litter, to represent the variety of substrates which microbes encounter in their natural environments (Mooshammer et al. 2014a). Hence, within each soil type, the C content of all substrate treatments was the same (30% of microbial biomass C), while the N and micronutrient contents changed at set ratios. We used the buffered Hoagland’s nutrient solution to avoid pH shifts (supplementary material 2) isolating the microbial response to substrate C:N from the effect of pH (Rousk et al. 2010) and eradicating potential micronutrient co-limitation (Liebig 1841).
Running the experiment
Subsamples of 50 g from each of the six field replicates of each of the eight soil types (n = 48 soils) were adjusted to 75% of maximum WHC using deionised water and equilibrated at 13 °C for 5 days prior to the experiment. Thereafter, five aliquots of 5 g fresh weight of each soil were transferred to 250 ml canning jars (n = 240 incubations). Each aliquot was amended with one of the five treatments: either one of the four substrate treatments (glu only, glu + N, glu + NN, glu + NNN) or deionised water as control. All substrate additions and water controls were pipetted as 1 ml liquid solution per replicate onto the relevant soil samples. Thereafter, jars were flushed with compressed air for 40 s, sealed, and over-pressurised by injecting 40 ml compressed air. All jars were then incubated in the dark for 21 days at 13 °C, which corresponds to the ambient mean summer temperature of both field sites (Whitaker et al. 2014; Parker et al. 2015).
Gas sampling for total CO2 analysis was conducted at t0 = 0 h, t1 = 24 h, t2 = 48 h, t3 = 168 h, t4 = 192 h, t5 = 336 h and t6 = 504 h after starting the experiment and samples for 13C analysis were taken at t1, t3, t4 and t6. To keep CO2 headspace concentrations in the jars below 10,000 ppm to avoid related feedbacks on soil respiration, the jars were opened after sampling at t3, then flushed, over-pressurised and sampled again as at the beginning of the experiment (t4). For analysis of total CO2 on the GC (Perkin Elmer Autosystem Gas Chromatograph, Speck & Burke, Alva, Clackmannanshire, Scotland), we took 5 ml sample air and transferred it to 3 ml evacuated exetainers (Labco, UK). For 13-C analysis, we took 20 ml sample air and transferred it to 12 ml exetainers before analysing them by cavity ring-down spectroscopy (CRDS) using a Picarro G2201i with a multiplexor (Picarro Inc., Santa Clara, California, USA). The accumulation of CO2 was assumed to be linear between the chosen time points for sampling as described above, so fluxes were expressed per hour and then corrected for CO2–C respired relative to respective soil C content, as this is also the unit chosen to express priming effects. Due to the loss of some samples, priming could not be calculated for all replicates of all soil types at all time points, with the final number of observations totalling n = 91 for Andean mountains and n = 88 for boreal subarctic.
Isotopic and source partitioning
The isotopic 13-C labelling of the added substrate solutions allowed the separation and quantification of CO2–C originating from native soil organic matter and from added glucose. In the following mass balance, RS represents the CO2 respired from soil, RG represents the CO2 originating from glucose and RT represents the total CO2 respired (Eq. 1). These can be separated into their respective sources (Eq. 2) with the known isotopic abundance of 13C of soil organic matter (13CS), glucose (13CG) and the total CO2-C respired (13CT). The priming effect, i.e. the substrate-induced change in the amount of C respired from soil (RPE), is quantified (Eq. 3) as the difference between soil organic matter-derived CO2-C respired from soils amended with glucose (RG) relative to the total amount of CO2-C respired from soil organic matter in the untreated control soils (RC):
The amount of primed C was then expressed as μg CO2-C g−1 soil C, in order to normalise for the differences in soil types and their C contents and increase comparability amongst the soils and their vulnerability to priming. The magnitude of priming (%) expresses the amount of primed C relative to the amount of C respired from untreated control soils. Substrate use (%) was calculated as the amount of added substrate-C detected in soil respiration, divided by the initial amount of substrate-C added to the soil and multiplied by 100.
Statistical analysis
Statistical analysis was carried out using R 4.0.5 (R Core Team 2021) with the additional packages of multcompView (Graves et al. 2015), PerformanceAnalytics (Peterson and Carl 2020) and betareg (Cribari-Neto and Zeileis 2010). First, we used repeated measure analysis of variance (ANOVA) to test the effect of incubation time, soil type and substrate treatment on soil respiration (Fisher 1925). To identify whether the observed priming effects were significantly different in the high altitudes of the Andean mountains and the high latitudes of the boreal subarctic we used Welch Two Sample t-test, which tests the hypothesis that two populations have equal means (Welch 1947). To compare relative microbial substrate use between the two regions we used Wilcoxon test which evaluates whether the data is symmetrically distributed around a specified median (Wilcoxon 1945). For both statistics, the significance level was set to 5% (α = 0.05). Three-way ANOVA was used to explain the distribution of the response parameters “priming effect” and “substrate use” by dint of the variables land cover type, soil horizon and substrate treatment respectively. Data were transformed to meet assumptions of linear regression where necessary. To unravel the role of N, we tested how soil, substrate and microbial biomass C:N affected priming applying three-way analysis of covariance (ANCOVA) with substrate C:N as categorical independent variable with four levels (each treatment) and microbial and soil C:N as covariates (Keppel 1982). To further identify potential drivers of priming effects, a multiple linear model was built for each data set (Peruvian high altitudes and Swedish high latitudes) using the explanatory variables of soil, substrate and microbial C and N contents. Models were simplified using backward stepwise selection according to the Akaike Information criterion (Akaike 1974). Linear regression and the Spearman’s rank correlation coefficient (Spearman 1904) were used to describe the relationship between substrate use and priming. Beta regression via maximum likelihood (Cribari-Neto and Zeileis 2010) was applied to determine potential drivers of substrate use, which had continuous values, but was bound between 0 and 100% of substrate possibly used by microbes.
Results
Soil and microbial characteristics
The studied soils represent a range of contrasting edaphic and microbial parameters (Table 1). Soil and microbial biomass C:N were lowest in Peruvian mineral forest soils (soil C:N = 15.3, microbial biomass C:N = 2.9) and highest in Swedish organic tundra soils (soil C:N = 34.7, microbial biomass C:N = 16). For each land cover type, soil and microbial C and N contents were higher in organic soils compared to their mineral counterparts. Mineral soil N was lower in Peruvian than in Swedish soils, and highest in Swedish organic soils. Physical soil parameters also covered a broad range, with maximum water holding capacity between 50.3 (BFM) and 463.2 (BFO), bulk density between 0.1 (AFO) and 0.93 (BTM) and predominant soil texture ranging from mostly organic matter (AFO, APO, BFO, BTO) to clay loam (AFM, APM) and sand (BFM, BTM). All soils were slightly acidic with pH between 4.2 (AFO) and 5.7 (BFM). Substrate-induced pH shifts were low and comparable for all soil and substrate combinations (on average reduced by 0.16 ± 0.11), with the greatest shifts in mineral forest soils from both regions (supplementary material 2).
Soil respiration
All soils responded to substrate additions with an initial peak in respiration (Fig. 1). This peak occurred 24 h after substrate addition in all Andean and in the mineral soils from Sweden and 48 h after substrate addition in the organic soils from Sweden (BFO, BTO), where peaks had the greatest magnitude (flux rates temporary tripled). Most control soils also showed moderate peaks in basal CO2-respiration within the first 48 h of incubation, as they were amended with 1 ml water at the beginning of the experiment in the same manner as substrate solutions were applied. In all cases, respiration rates of the treated soils stabilised after 48 h at the level of the respective control soils, with the respiration in the initial 48 h being significantly different from the later incubation (p < 0.001). On average, respiration rates from subarctic soils were 4–20 times higher than respiration from the Andean soils (p = 0.002), with overall highest respiration in organic and mineral boreal forest soils (BFO, BFM). In both the Andean and the subarctic regions, mineral soils from above the treeline (APM, BTM) had the lowest respiration rates, followed by on average two-fold higher respiration from corresponding organic soils (APO, BTO). Similarly, mineral forest soil respiration (AFM, BFM) was half as high as respiration measured from corresponding organic forest soils (AFO, BFO). These differences between soil types were statistically significant between and within both regions at all time points (p < 0.001).
Soil respiration (μg CO2–C per unit of g soil C per hour) from the eight treeline soils studied Aliquots of each soil were amended with substrates, which all contained glucose (glu) as C source, while three treatments also contained nitrogen (N) at different concentrations (C:N 7:1, 17:1 and 71:1), and one aliquot per replicate per soil type was kept as control and amended with water (n = 6). Respiration was measured for 504 h (21 days) in controlled laboratory soil incubations. Please note different scale on y-axis. One panel per soil type: Andean Forest Organic (AFO), Andean Forest Mineral (AFM), Andean Puna Organic (APO), Andean Puna Mineral (APM), Boreal Forest Organic (BFO), Boreal Forest Mineral (BFM), Boreal Tundra Organic (BTO), Boreal Tundra Mineral (BTM)
Soil C priming and substrate use
Direction and magnitude of priming varied amongst the different treeline soils with equal observations of positive and negative priming (Fig. 2 left panels). Priming effects were significantly different in the Andean soils and the boreal subarctic soils (p = 0.03), with the absolute amounts of primed C (μg C (g soil C) −1) ten times higher in the subarctic soils, notably the forest soils (Fig. 2a, e). The magnitude of priming was however similar in both regions (p = 0.6), with most absolute values between 10 and 35% (Fig. 2c, g). In the Andean mountain soils (Fig. 2a, c), primed C was statistically different between land cover types (p = 0.007), with predominantly negative priming in forest soils (AFO, AFM) and more positive priming in Puna grassland soils (APO, APM). For the subarctic soils (Fig. 2e, g), significant differences in priming effects were observed between soil horizons (p < 0.001), with mostly positive priming in mineral soils (BFM, BTM) and predominantly negative priming in organic soils of both forest (BFO) and tundra (BTO). The magnitude of priming was significantly higher in forest soils (BFO, BFM) compared to tundra soils (BTO, BTM). Within each land cover type, priming was higher in mineral soils compared to their organic counterparts.
Priming effects and substrate use in Andean (a–d) and boreal (e–h) treeline soils. Left panels show absolute priming effects [(µg C) (g soil C)−1] and magnitude of priming (%) and right panels show absolute [(µg C) (g soil C)−1] and relative (%) substrate use. Each soil was amended with four substrate treatments, all of which contained glucose (glu) as C source, and three contained also nitrogen (N) at different concentrations (C:N 7:1, 17:1 and 71:1), as indicated in the upper right panels for each region. Boxes show median lines and interquartile ranges. Statistically significant results of three-way ANOVA testing for correlation between each of the observed variables (priming effect or substrate use) and the explanatory variables (land cover, soil horizon and treatment) are provided in each plot with asterisk indicating the significance level at < 0.001 ‘***’ ≤ 0.01 ‘**’ ≤ 0.05 ‘*’. The soil types are Andean Forest Organic (AFO), Andean Forest Mineral (AFM), Andean Puna Organic (APO), Andean Puna Mineral (APM), Boreal Forest Organic (BFO), Boreal Forest Mineral (BFM), Boreal Tundra Organic (BTO), Boreal Tundra Mineral (BTM)
Substrate use (Fig. 2 right panels) was significantly higher in the Andean soils compared to the boreal subarctic soils (p < 0.001). In the Andean soils (Fig. 2b, d) substrate use was significantly higher (p < 0.001) in organic (AFO, APO) compared to mineral soils (AFM, APM), with a significant interaction with substrate treatment regarding the relative amounts of substrate used (p = 0.02). In the organic soils, relative substrate use was highest for the C-only addition, while in mineral soils it was highest for substrate additions with a C:N of 17:1 (Fig. 2d). In the subarctic soils (Fig. 2f, h) substrate use was significantly higher (p < 0.001) in the organic (BFO, BTO) than in the mineral soils (BFM, BTM), with larger quantities used in the forest compared to the tundra soils (p < 0.001).
Microbial substrate use was inversely correlated with the magnitude of priming in most of the soils (Fig. 3), notably in the Andean forest soils (AFO, AFM) and the organic soils from the subarctic (BFO, BTO). Consistent amongst these soils, increased substrate use decreased priming effects, a relationship which was supported in the linear models (Table 4).
Relationship between substrate-use (%) and magnitude of priming (%) in the eight soil types studied. Plots show individual data points, the resistant line in black, the least square line in red and p and r values. Strongest correlation (p < 0.1 ˄ r > 0.45) in organic soils from the subarctic (BFO and BTO) and forest soils of the Andes (AFO and AFM). The least square regression line makes the vertical distance from each data point to the regression line as small as possible, while for the resistant line, the median residual in each outer third of the data is zero, so that it is resistant to outliers
Effect of N-availability on priming
We conducted an ANCOVA to investigate the interactive effects of soil, substrate and microbial biomass C:N on priming and found that soil C:N was a significant covariate determining the magnitude of priming effects in 7 of the 8 soils studied (Table 3). This finding is in line with the spatially explicit patterns observed in priming effects and microbial substrate use, which were also distinctive according to land cover types and organic and mineral soil horizons (Fig. 2), which are inherently linked to the different soil C:N (Table 1). In addition, soil mineral N was significant in determining substrate use in the Andean soils, which in turn determined priming effects (Table 4). In the subarctic soils, microbial biomass N also determined microbial substrate use, along with microbial biomass C, soil C and substrate C (Table 4). Substrate C:N, and therewith exogenous N-availability, was mostly not linked to the observed priming effects (Table 3), but had a significant effect in the organic boreal soils (BFO), where priming decreased with increasing added N content (Fig. 2e). In the mineral forest soils of both geographic regions (AFM, BFM), the interaction between soil and substrate C:N also had a significant effect on priming, as well as the interaction between soil and microbial biomass C:N (Table 3). The three-way interaction and the interaction between microbial biomass C:N and substrate C:N was not significant in any soil.
Discussion
In a direct comparison using standardised methodology for soils from high altitudinal and high latitudinal ecosystems, we provide new insights to the mechanisms of priming effects and their potential ecological relevance in the context of sensitive ecosystems under climate change.
Preferential substrate use decreases priming effects
Negative priming occurred in half of the studied soils in both ecosystems (Fig. 2). Inverse correlations between the magnitude of priming and microbial substrate use support our hypothesis that preferential use of the added substrate decreased rates of SOM-mineralisation (H2), contributing to the negative priming effects observed (Fig. 3). In recent literature, negative priming received less attention than positive priming, even though negative priming is an important process across ecosystems (Guenet et al. 2010; Blagodatskaya et al. 2011; Bastida et al. 2019; Siles et al. 2022). Our results indicate that preferential substrate use is one of the key drivers of negative priming effects (Cheng and Kuzyakov 2005; Blagodatskaya et al. 2007; Wang et al. 2015). Accordingly, it has been shown that preferential substrate use can be a beneficial microbial strategy of resource acquisition, reducing the investment into SOM-degrading enzymes (Sinsabaugh et al. 2016; Merino et al. 2016; Amenabar et al. 2017). Preferential substrate use and negative priming are likely constantly occurring in ecosystems, particularly in immediate plant-soil-microbe interactions in the rhizosphere where root exudates provide a range of substances with variable C:N and many sugars to microbes (Jones et al. 2009; Gunina and Kuzyakov 2015; Canarini et al. 2019). Preferential substrate use could therefore also shape C cycling at the ecosystem scale. For example, in a large-scale warming experiment in the UK, Briones et al. (2021) found no evidence for positive priming, while enhanced turnover of recently fixed C suggests that preferential substrate use can take place at the landscape scale.
While substrate use was mostly independent from substrate C:N, it was idiosyncratic of land cover type and soil horizon (Fig. 2 right panels), in line with the observed priming effects (Fig. 2 left panels). Microbial substrate use explained particularly the negative priming effects in this study (Fig. 3, Table 4), thus supporting the central role of microbes in the soil C cycle (Kuzyakov 2010; Cortufo et al. 2013; Classen et al. 2015; Liang et al. 2017; Kyker-Snowman et al. 2020). However, we did not observe that substrate use was highest when the C:N composition of the substrate matched that of the receiving microbial community, and the interaction term between substrate C:N and microbial biomass C:N was not a significant predictor of priming effects (Table 3). Microbes are hence not generally more likely to utilize substances when they match their biomass C:N. Our results rather indicate that there may be more complex stochiometric linkages. For example, microbes had a higher substrate use for substrate with a C:N of 17:1 in the Andean mineral soils, and in organic soils substrate use was highest when only C was supplied (Fig. 2d). This observation could indicate different functional capacities of different microbial communities (Kaiser et al. 2010; Krause et al., 2014), which could enable the prediction of substrate use based on a metabolic profile for respective microbial communities.
No support for microbial N-mining
Soil C and mineral N determined substrate use in the Andes, which in turn determined priming effects, suggesting an indirect relationship between priming and soil mineral N (Table 4). However, there was not more positive priming in the soils of lowest N-availability, nor decreased positive priming when more N was added. These results thus do not support the N-mining hypothesis, which is in line with other studies (Mason-Jones et al. 2018; Wild et al. 2019). Nonetheless, microbial N-mining remains an intriguing principle (Craine et al. 2007; Chen et al. 2014; Hicks et al. 2020), but it seems to apply only when the N obtainable from bioavailable sources (e.g. exogenous organic substrates, microbial necromass) is insufficient to meet an increased microbial nutrient demand and when adjustment of microbial C and N use efficiencies and stoichiometry cannot compensate for temporal C or N shortages (Cotrufo et al. 2013; Mooshammer et al. 2012, 2014b; Spohn 2016; Averill and Waring 2017). It is therewith not surprising to find no support for N-mining at the low rates of substrate added in this study. Microbial N-mining is more likely to occur when the active microbial community grows or its composition changes (Fontaine et al. 2003; Mondini et al. 2006; Li et al. 2018; Salazar et al. 2019). Greater amounts of N released from the soil matrix can also occur as the result of purely physio-chemical interactions between soil particles and root exudates (e.g. oxalic acid) or pH shifts in the rhizosphere (Rousk et al. 2010; Keiluveit et al. 2015).
Microbial stoichiometric flexibility at low levels of substrate addition
Another novelty of this study is that we show preferential substrate use at low levels of substrate additions (30% of microbial biomass C), while it was previously assumed to occur when substrate additions equal or exceed the C content of microbial biomass in soil by 50–1200% (Blagodatskaya and Kuzyakov 2008). We suggest that C addition rates below 50% of microbial biomass C fall within the ecological threshold within which microbial plasticity can buffer against the stoichiometric variability of the exogenously supplied substrate (Rousk et al. 2015; Spohn 2016; Buckeridge and McLaren 2020; Camenzind et al. 2020). Thus, the C:N of the added substrate as such is less deterministic for soil C cycling rates, which might be better accessed through microbial C and N use efficiencies (Manzoni et al. 2012; Mooshammer et al. 2014a, b; Kyker-Snowman et al. 2020; Soares and Rousk 2019). This interpretation is in accordance with a study on grassland soils, which showed that priming effects can be disentangled from even larger gradients of N-additions through plasticity of microbial C use efficiency (Zhang et al. 2020) and with a study demonstrating highly flexible C:N:P ratios in fungal hyphae (Camenzind et al. 2020). Stochiometric flexibility may also explain why we found no support for the hypothesis that substrate use would be highest, and priming lowest, when the C:N of inputs matched that of microbial biomass (Table 3). Establishing a direct link between the stoichiometry of added substrate and microbial soil C and N mineralisation rates can be further impeded when microbes selectively target soil resources of a higher nutritional value, for example with high N-content. In this case, microbial nutrient uptake can be enhanced while the cost—and loss—of C is reduced. This mechanism of "selective N-targeting” (Murphy et al. 2015; Rousk et al. 2016), which is effectively a form of C-neutral N-mining, can explain reduced rates of SOM-mineralisation and CO2-respiration, despite increased microbial N demand and uptake (Fig. 4).
Conceptual framework of the mechanisms of positive and negative priming effects and their interlinkages. The two classic pathways described in the literature are (i) Microbial N-mining: Microbes use substrate-C as energy to obtain N from SOM, which increases mineralisation rates (positive priming effect), leading to net soil carbon loss, (ii) Preferential substrate use: The added substrate matches the microbial demand, so they switch energy and nutrient acquisition from soil sources to substrate, reducing rates of SOM-mineralisation (negative priming effect), leading to no change in the C balance. For C gain, and eventually C sequestration, the net inputs need to exceed the outputs, which is the case if (i) microbial biomass C increases substantially and/or (ii) a significant proportion of the initially added substrate is not metabolised at all. It is also possible that microbes use the added substrate as energy source to specifically degrade SOM rich in N (selective N-targeting). C carbon, N nitrogen, SOM soil organic matter
Potential ecological implications
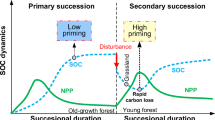
Species range shifts have been reported for various montane and alpine ecosystems around the world, though the tropical treeline has so far remained relatively stable (Kramer et al. 2009; Harsch et al. 2009; Rehm and Feeley 2015). The direction of priming was consistent with our first hypothesis (H1) in the Andes, where we observed positive priming in Puna grasslands above the treeline and negative priming in the soils of the Andean mountain forest (Fig. 2a, c). If climate change causes large scale range shifts of plant communities in mountain systems like the Andes, consistently accompanied by positive priming effects as observed in the Puna soils of this and other studies (Yang et al. 2018), the disproportionally increased rates of soil C-mineralisation have the potential to disturb the C balance of these ecosystems with unknown consequences for atmospheric CO2 concentrations.
On the other hand, the treeline at the Abisko study site has shifted northwards in recent years (Wookey et al. 2009). Several studies indicate that this may accelerate C turnover and thus link to the lower soil C stocks in the soils of the birch forests (Hartley et al. 2010; Parker et al. 2015; Keuper et al. 2020). We found that in these soils, soil horizon determined the direction of priming under both land cover types, with negative priming in organic soils and positive priming in mineral soils, in line with other studies (Hartley et al. 2010; Heitkötter et al. 2017). These results confirm that increasing organic inputs through changes in land cover and plant community composition might disproportionally stimulate microbes in the deeper soil horizons, causing additional C losses from these soils through positive priming effects (Fontaine et al. 2007). The re-distribution of plant-fixed C through the soil profile by deeper rooting plant species could therefore be an important factor in regulating the C balance in the future. Carbon losses from deeper soil layers may become more evident if warming soils and thawing permafrost facilitate deeper rooting of plant species above the treeline and labile inputs to soils increase due to higher plant productivity in a greening Arctic.
Scalability and outlook
To obtain realistic estimates of the impact of priming effects on the study system, it is important to also account for the amount of exogenous C which was added, but not metabolised by microbes at all (Fig. 4; Dalenberg and Jager 1980; Gunina et al. 2014). When the amount of experimentally added C exceeds the amount of additional C respired from SOM, C losses caused by positive priming can be cancelled out or even reversed (Qiao et al. 2014; Liang et al. 2018; Perveen et al. 2019). Consistently, also in this study the C inputs to soils exceed the C outputs in all cases, even when positive priming is reported, highlighting a common misconception: Positive soil carbon priming is not equal to net soil carbon loss. It is fallacious to conclude net soil C loss from positive priming, or from N-mining per se, as several processes can result in a neutral or even negative soil C balance (Fig. 4). In addition, priming effects are seldomly measured in situ and across geographic and temporal scales, making it difficult to integrate and upscale short-lived priming effects to ecosystems, where several factors influence microbial activity, which are not captured in laboratory soil incubations (Cardinael et al. 2015). To bridge the gap between reductionist laboratory approaches and modelling the complexity of C cycling in natural ecosystems, it could be helpful to further conceptualise the resource acquisition strategies of different soil microbial communities and quantify the C inputs and nutrient uptakes of different plant species and communities (Kuzyakov and Domanski 2000; Kaiser et al. 2011; Krause et al. 2014; Shahzad et al. 2015; Guyonnet et al. 2018). Identifying the parameters needed to predict priming effects at ecosystem scale would help to better understand the impact of climate change on the global C cycle and make informed decisions about land use (Jenny 1980).
Conclusions
In Puna grassland soils of the high altitudinal Andean mountains and in mineral soils from the high latitudinal subarctic, rates of SOM-mineralisation increased after substrate additions (positive priming). If we expect large-scale upwards shifts of treelines in the Andes and deeper rooting plants in a greening Arctic, the enhanced C inputs and positive priming effects could translate to disproportionate C losses from the C-rich soils in these regions. However, in our experimental set-up the amount of C lost through positive priming was lower than the amount of C added at the beginning of the experiment (no net C loss). Moreover, negative priming effects were also frequently observed and consistently correlated with increased microbial substrate use. Preferential substrate use as a key driver of negative priming could contribute to the accumulation of organic C in soils over time. To better understand the true impact of priming effects on the C balance of ecosystems, it will be an important step to test the mechanistic insights gained in this and other laboratory studies at scales that account for the diurnal and seasonal variability of organic inputs in natural ecosystems with live plants, which act also as a major sink of nutrients. A better understanding of the mechanisms of priming effects in situ could help to reduce uncertainty in quantifying the impact of climate change induced species shifts on soil C stocks, improve process-based models of ecosystem C cycling and clarify C sequestration potential of soils.
Data availability
All data generated during the current study are presented in the manuscript and available from the authors.
References
Akaike H (1974) A new look at the statistical model identification. IEEE Trans Autom Control 19(6):716–723. https://doi.org/10.1109/TAC.1974.1100705
Amenabar MJ, Shock EL, Roden EE, Peters JW, Boyd ES (2017) Microbial substrate preference dictated by energy demand rather than supply. Nat Geosci 10:577–581
Averill C, Waring B (2017) Nitrogen limitation of decomposition and decay: how can it occur? Glob Change Biol 24:1417–1427
Bastida F, Garcia C, Fierer N, Eldridge DJ, Bowker MA, Abades S, Alfaro FD, Berhe AA, Cutler NA, Gallardo A, Garcia-Velazquez L, Hart SC, Hayes PE, Hernandez T, Hseu Z-Y, Jehmlich N, Kirchmair M, Lambers H, Neuhauser S, Pena-Ramirez VM, Perez CA, Reed SC, Santos F, Siebe C, Sullivan BW, Trivedi P, Vera A, Williams MA, Moreno JL, Delgado-Baquerizo M (2019) Global ecological predictors of the soil priming effect. Nat Commun. https://doi.org/10.1038/s41467-019-11472-7
Berdanier AB (2010) Global treeline position. Nat Educ Knowl 3(10):11
Bingeman CW, Varner JE, Martin WP (1953) The effect of the addition of organic materials on the decomposition of an organic soil. Soil Sci Soc Am J 17:34–38
Blagodatskaya EV, Kuzyakov Y (2008) Mechanisms of real and apparent priming effect and their dependence on soil microbial biomass and community structure: critical review. Biol Fertil Soils 45:115–131
Blagodatskaya EV, Blagodatsky SA, Anderson TH, Kuzyakov Y (2007) Priming effects in chernozem induced by glucose and N in relation to microbial growth strategies. Appl Soil Ecol 37:95–105
Blagodatskaya EV, Yuyukina T, Blagodatsky S, Kuzyakov Y (2011) Turnover of soil organic matter and of microbial biomass under C3–C4 vegetation change: consideration of 13C fractionation and preferential substrate utilization. Soil Biol Biochem 43(1):159–166
Blagodatsky S, Blagodatskaya EV, Yuyukina T, Kuzyakov Y (2010) Model of apparent and real priming effects: linking microbial activity with soil organic matter decomposition. Soil Biol Biochem 42:1275–1283
Briones MJI, Garnett MH, Ineson P (2021) No evidence for increased loss of old carbon in a temperate organic soil after 13 years of simulated climatic warming despite increased CO2 emissions. Glob Change Biol 27(9):1836–1847. https://doi.org/10.1111/gcb.15540
Buckeridge KM, Zufelt E, Chu H, Grogan P (2010) Soil nitrogen cycling rates in low arctic shrub tundra are enhanced by litter feedbacks. Plant Soil 330:407–421
Buckeridge KM, McLaren JR (2020) Does plant community plasticity mediate microbial homeostasis? Ecol Evol 10(12):5251–5258
Camenzind T, Grenz KP, Lehmann J, Rillig MC (2020) Soil fungal mycelia have unexpectedly flexible stochiometric C: N and C: P ratios. Ecol Lett 24(2):208–218
Canarini A, Kaiser C, Merchant A, Richter A, Wanek W (2019) Root exudation of primary metabolites: mechanisms and their roles in plant responses to environmental stimuli. Front Plant Sci 10:157. https://doi.org/10.3389/fpls.2019.00157
Cardinael R, Eglin T, Guenet B, Neill C, Houot S, Chenu C (2015) Is priming effect a significant process for long-term SOC dynamics? Analysis of a 52-years old experiment. Biogeochemistry 123:203–219
Chen R, Senbayram M, Blagodatsky S, Myachina O, Dittert K, Lin X, Blagodatskaya E, Kuzyakov Y (2014) Soil C and N availability determine the priming effect: microbial N mining and stoichiometric mineralization theories. Glob Change Biol 20:2356–2367
Cheng W (1999) Rhizosphere feedbacks in elevated CO2. Tree Physiol 19:313–320
Cheng W, Kuzyakov Y (2005) Root effects on soil organic matter decomposition. In: Zobel RW, Wright SF (eds) Roots and soil management: interactions between roots and the soil. American Society of Agronomy Crop Science Society of America Soil Science Society of America, Madison. https://doi.org/10.2134/agronmonogr48.c7
Classen AT, Sundqvist MK, Henning JA, Newman GS, Moore JAM, Cregger MA, Moorhead LC, Patterson CM (2015) Direct and indirect effects of climate change on soil microbial and soil microbial-plant interactions: what lies ahead? Ecosphere 6(8):130
Cotrufo MF, Wallenstein MD, Boot CM, Denef K, Paul E (2013) The microbial efficiency-matrix stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: do labile plant inputs form stable soil organic matter? Glob Change Biol 19:988–995
Craine JM, Morrow C, Fierer N (2007) Microbial nitrogen limitation increases mineralization. Ecology 88:2105–2113
Cribari-Neto F, Zeileis A (2010) Beta regression in R. J Stat Softw. https://doi.org/10.18637/jss.v034.i02
Dalenberg JW, Jager G (1980) Priming effect of small glucose additions to 14C-labelled soil. Soil Biol Biochem 13:219–223
Davis ROE, Bennett HH (1927) Grouping of soils on the basis of mechanical analysis. U.S. Department of Agriculture, Washington, D.C. https://naldc.nal.usda.gov/download/CAT87217164/pdf. Accessed 3 Apr 2022
Dijkstra FA, Carrillo Y, Pendall E, Morgan JA (2013) Rhizosphere priming: a nutrient perspective. Front Microbiol. https://doi.org/10.3389/fmicb.2013.0021
Emmett BA, Frogbrook ZL, Chamberlain PM, Griffiths R, Pickup R, Poskitt J, Reynolds B, Rowe, Spurgeon ED, Rowland P, Wilson J, Wood CM (2008) Soils manual. CS Technical Report 3/07. Centre for Ecology and Hydrology, Natural Environmental Research Council, Wallingford, UK
Feeley KJ, Bravo C, Fadrique B, Perez T, Zuleta D (2020) Climate-driven changes in the composition of New World plant communities. Nat Clim Chang. https://doi.org/10.1038/s41558-020-0873-2
Fierer N, Schimel J (2003) A proposed mechanism for the pulse in carbon dioxide production commonly observed following the rapid rewetting of a dry soil. Soil Sci Soc Am J 67:798–805
Fisher JB, Malhi Y, Cuba Torres I, Metcalf DB, van de Weg MJ, Meir P, Silva-Espejo JE, Huaraca Huasco W (2013) Nutrient limitation in rainforests and cloud forests along a 3,000-m elevation gradient in the Peruvian Andes. Oecologia 172:889–902
Fisher RA (1925) Statistical methods for research workers. Oliver and Boyd, Edinburgh, UK. https://doi.org/10.1111/j.2397-2335.1926.tb01837.x
Fontaine S, Mariotti A, Abbadie L (2003) The priming effect of organic matter: a question of microbial competition? Soil Biol Biochem 35(6):837–843
Fontaine S, Barot S, Barré P, Bdioui N, Mary B, Rumpel C (2007) Stability of organic carbon in deep soil layers controlled by fresh carbon supply. Nature 450:277–280
Food and Agriculture Organization of the United Nations (FAO) (1971) Soil map of the world, Volume IV South America, Unesco Paris. https://www.fao.org/3/as361s/as361s.pdf. Accessed 3 Apr 2022
Food and Agriculture Organization of the United Nations (FAO) (2015) World reference base for soil resources 2014, International soil classification system for naming soils and creating legends for soil maps, Update 2015, ISSN 0532–0488
Girardin CAJ, Malhi Y, Doughty CE, Metcalfe DB, Meir P, Aguila-Pasquel J, Araujo-Murakami A, da Costa ACL, Silva Espejo JE, Amézquita FF, Rowland L (2016) Seasonal trends of Amazonian rainforest phenology, net primary productivity and carbon allocation. Glob Biogeochem Cycles 30(5):700–715
Graves S, Piepho HP, Selzer L, Dorai-Raj S (2015) multcompView: visualizations of paired comparisons. R package version 0.1–7. http://CRAN.R-project.org/package=multcompView. Accessed 3 Apr 2022
Gregorich EG, Wen G, Voroney RP, Kachanoski RG (1990) Calibration of a rapid direct chloroform extraction method for measuring soil microbial biomass C. Soil Biol Biochem 22(7):1009–1011
Guenet B, Neill C, Bardoux G, Abbadie L (2010) Is there a linear relationship between priming effect intensity and the amount of organic matter input? Appl Soil Ecol 46:436–442
Gunina A, Dippold M, Glaser B, Kuzyakov Y (2014) Fate of low molecular weight organic substances in an arable soil: from microbial uptake to utilisation and stabilisation. Soil Biol Biochem 77:304–313
Gunina A, Kuzyakov Y (2015) Sugars in soil and sweets for microorganisms: review of origin, content, composition and fate. Soil Biol Biochem 90:87–100
Guyonnet JP, Canatarel AA, Simon L, el Zahar Haichar F (2018) Root exudation rate as functional trait involved in plant-nutrient-use strategy classification. Ecol Evol 8(16):8573–8581
Halbritter AH, De Boeck HJ, Eycott AE et al (2020) The handbook for standardised field and laboratory measurements in terrestrial climate-change experiments and observational studies (ClimEx). S2: Carbon and nutrient cycling; protocol 2.2.1 Soil microbial biomass – C, N, and P (Schmidt IK, Reinsch S, Christiansen CT). Methods Ecol Evol 11(1):22–37. https://doi.org/10.1111/2041-210X.13331
Harsch MA, Hulme PE, McGlone MS, Duncan RP (2009) Are treelines advancing? A global meta-analysis of treeline response to climate warming. Ecol Lett 12:1040–1049
Hartley IP, Hopkins DW, Sommerkorn M, Wookey PA (2010) The response of organic matter mineralisation to nutrient and substrate additions in subarctic soils. Soil Biol Biochem 42:92–100
Hartley IP, Garnett MH, Sommerkorn M, Hopkins DW, Fletcher BJ, Sloan VL, Wookey PA (2012) A potential loss of carbon associated with greater plant growth in the European Arctic. Nat Clim Chang 2:875–879
Heitkötter J, Heinze S, Marschner B (2017) Relevance of substrate quality and nutrients for microbial C-turnover in top and subsoil of a Dystric Cambisol. Geoderma 302:89–99
Hicks LC, Leizeaga A, Rousk K, Michelsen A, Rousk J (2020) Simulated rhizosphere deposits induce microbial N-mining that may accelerate shrubification in the subarctic. Ecology. https://doi.org/10.1002/ecy.3094
Jenny H (1980) Alcohol or humus? Science 209:444
Jones DL, Nguyen C, Finlay RD (2009) Carbon flow in the rhizosphere: carbon trading at the soil–root interface. Plant Soil 321:5–33
Kaiser C, Koranda M, Kitzler B, Fuchslueger L, Schnecker J, Schweiger P, Rasche F, Zechmeister S, Zechmeister-BoltensternRichter A (2010) Belowground carbon allocation by trees drives seasonal patterns of extracellular enzyme activities by altering microbial community composition in a beech forest soil. New Phytol 187(3):843–858
Kaiser C, Fuchslueger L, Koranda M, Gorfer M, Stange CF, Kitzler B, Rasche F, Strauss J, Sessitsch A, Zechmeister-Boltenstern S, Richter A (2011) Plants control the seasonal dynamics of microbial N cycling in a beech forest soil by belowground C allocation. Ecology 92(5):1036–1051
Keiluveit M, Bougoure JJ, Nico PS, Pett-Ridge J, Weber PK, Kleber M (2015) Mineral protection of soil carbon counteracted by root exudates. Nat Clim Chang 5(6):588–595
Keppel G (1982) Design and analysis: a researcher’s handbook. Prentice-Hall, Englewood Cliffs
Keuper F, Wild B, Kummu M, Beer C, Blume-Werry G, Fontaine S, Gavazov K, Gentsch N, Guggenberger G, Hugelius G, Jalava M, Koven C, Krab EJ, Kuhry P, Monteux S, Richter A, Shahzad T, Weedon JT, Dorrepaal E (2020) Carbon loss from northern circumpolar permafrost soils amplified by rhizosphere priming. Nat Geosci 13:560–565
Körner C, Paulsen J (2014) A climate-based model to predict potential treeline position around the globe. Alp Bot 124(1):1–12
Kramer A, Hagedorn F, Shevchenko I, Leifeld J, Guggenberger G, Goryacheva T, Rigling A, Moiseev P (2009) Treeline shifts in the Ural mountains affect soil organic matter dynamics. Glob Change Biol 15:1570–1583
Krause S, Le Roux X, Niklaus PA, Van Bodegom PM, Lennon JT, Bertilsson S, Grossart H-P, Philippot L, Bodelier PLE (2014) Trait-based approaches for understanding microbial biodiversity and ecosystem functioning. Front Microbiol 5:251. https://doi.org/10.3389/fmicb.2014.0025
Kuzyakov Y (2010) Priming effects: interactions between living and dead organic matter. Soil Biol Biochem 42:1363–1371
Kuzyakov Y, Cheng W (2001) Photosynthetic controls of rhizosphere respiration and organic matter decomposition. Soil Biol Biochem 33:1915–1925
Kuzyakov Y, Domanski G (2000) Carbon inputs by plants into the soil. Review. J Plant Nutr Soil Sci 163:421–431
Kuzyakov Y, Friedel JK, Stahr K (2000) Review of mechanisms and quantification of priming effects. Soil Biol Biochem 32:1485–1498
Kyker-Snowman E, Wieder WR, Frey S, Grandy AS (2020) Stoichiometrically coupled carbon and nitrogen cycling in the microbial-mineral carbon stabilisation model (MIMICS-CN) geoscientific model development. Geosci Model Dev 13:4413–4434. https://doi.org/10.5194/gmd-2019-320
Lange S, Rockel B, Volkholz J, Bookhagen B (2015) Regional climate model sensitivities to parametrizations of convection and non-precipitating subgrid-scale clouds over South America. Clim Dyn 44(9–10):2839–2857
Li LJ, Zhu-Barker X, Ye R, Doane TA, Horwath WR (2018) Soil microbial biomass size and soil carbon influence the priming effect from carbon inputs depending on nitrogen availability. Soil Biol Biochem 119:41–49
Liang C, Schimel JP, Jastrow JD (2017) The importance of anabolism in microbial control over soil carbon storage. Nat Microbiol 2(8):17105
Liang J, Zhou Z, Huo C et al (2018) More replenishment than priming loss of soil organic carbon with additional carbon input. Nat Commun 9:3175. https://doi.org/10.1038/s41467-018-05667-7
Liebig J (1841) Organic chemistry in its application to agriculture and physiology. John Owen, Cambridge, England
Löhnis F (1926) Nitrogen availability of green manure. Soil Sci 22:253–290
Manzoni S, Taylor P, Richter A, Porporato A, Agren G (2012) Environmental and stoichiometric controls on microbial carbon-use efficiency in soils. New Phytol 196(1):79–91
Mason-Jones K, Schmücker N, Kuzyakov Y (2018) Contrasting effects of organic and mineral nitrogen challenge the N-mining hypothesis for soil organic matter priming. Soil Biol Biochem 124:38–46
Merino C, Godoy R, Matus F (2016) Soil enzymes and biological activity at different levels of organic matter stability. J Soil Sci Plant Nutr 16(1):14–30
Mondini C, Cayuela ML, Sanchez-Monedero MA, Roig A, Brookes PC (2006) Soil microbial biomass activation by trace amounts of readily available substrate. Biol Fertil Soils 42:542–549
Mooshammer M, Wanek W, Schnecker J, Wild B, Leitner S, Hofhansl F, Blöchl A, Hämmerle I, Frank AH, Fuchslueger L, Keiblinger KM, Zechmeister-Boltenstern S, Richter A (2012) Stoichiometric controls of nitrogen and phosphorus cycling in decomposing beech leaf litter. Ecology 93(4):770–782
Mooshammer M, Wanek W, Zechmeister-Boltenstern S, Richter A (2014a) Stoichiometric imbalances between terrestrial decomposer communities and their resources: mechanisms and implications of microbial adaptations to their resources. Front Microbiol 5:22. https://doi.org/10.3389/fmicb.2014.00022
Mooshammer M, Wanek W, Hämmerle I, Fuchslueger L, Hofhansl F, Knoltsch A, Schnecker J, Takriti M, Watzka M, Wild B, Keiblinger KM, Zechmeister-Boltenstern S, Richter A (2014b) Adjustment of microbial nitrogen use efficiency to carbon: nitrogen imbalances regulates soil nitrogen cycling. Nat Commun 5:3694
Murphy CJ, Baggs EM, Morley N, Wall DP, Paterson E (2015) Rhizosphere priming can promote mobilisation of N-rich compounds from soil organic matter. Soil Biol Biochem 81:236–243
Nottingham AT, Turner BL, Chamberlain PM, Stott AW, Tanner EVJ (2012) Priming and microbial nutrient limitation in lowland tropical forest soils of contrasting fertility. Biogeochemistry 111:219–237
Nottingham AT, Meir P, Velasquez EL, Turner B (2020) Soil carbon loss by experimental warming in a tropical forest. Nature 584(7820):234–237
Parker TC, Subke J-A, Wookey PA (2015) Rapid carbon turnover beneath shrub and tree vegetation is associated with low soil carbon stocks at a subarctic treeline. Glob Change Biol 21:2070–2081
Parker TC, Thurston AM, Raundrup K, Subke JA, Wookey PA, Hartley IP (2021) Shrub expansion in the Arctic may induce large-scale carbon losses due to changes in plant-soil interactions. Plant Soil. https://doi.org/10.1007/s11104-021-04919-8
Perveen N, Barot S, Alvarez G, Klumpp K, Martin R, Rapaport A, Herfurth D, Louault F, Fontaine S (2014) Priming effect and microbial diversity in ecosystem functioning and response to global change: a modelling approach using the SYMPHONY model. Glob Change Biol 20:1174–1190
Perveen N, Barot S, Maire V, Cotrufo FM, Shahzad T, Blagodatskaya E, Stewarth CE, Ding W, Siddiq MR, Dimassi B, Mary B, Fontaine S (2019) Universality of priming effect: an analysis using thirty-five soils with contrasted properties sampled from five continents. Soil Biol Biochem 134:162–171
Peterson BG, Carl P (2020) Performance analytics: econometric tools for performance and risk analysis, R package version 2.0.4. https://CRAN.R-project.org/package=PerformanceAnalytics
Qiao N, Schaefer D, Blagodatskaya E, Zou XM, Xu XL, Kuzyakov Y (2014) Labile-carbon retention compensates for CO2 released by priming in forest soils. Glob Change Biol 20:1943–1954
Qiao N, Xu X, Hu Y, Blagodatskaya E, Liu Y, Schaefer D, Kuzyakov Y (2016) Carbon and nitrogen additions induce distinct priming effects along an organic-matter decay continuum. Nat Sci Rep 6:19865. https://doi.org/10.1038/srep19865
R Core Team (2021) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Rehm EM, Feeley KJ (2015) The inability of tropical cloud forest species to invade grasslands above treeline during climate change: potential explanations and consequences. Ecography 38:001–009
Rolando JL, Turin C, Ramírez D, Mares V, Monerris J, Quiroz R (2017) Key ecosystem services and ecological intensification of agriculture in the tropical high-Andean Puna as affected by land-use and climate changes Agriculture. Ecosyst Environ 236:221–233
Rousk J, Brookes PC, Baath E (2010) Investigating the mechanisms for the opposing pH-relationships of fungal and bacterial growth in soil. Soil Biol Biochem 42:926–934
Rousk J, Hill PW, Jones DL (2015) Priming of the decomposition of ageing soil organic matter: concentration dependence and microbial control. Funct Ecol 29:285–296
Rousk K, Michelsen A, Rousk J (2016) Microbial control of soil organic matter mineralization responses to labile carbon in subarctic climate change treatments. Glob Change Biol 22:4150–4161
Saatchi SS, Harris NL, Brown S, Lefsky M, Mitchard ET, Salas W, Zutta BR, Buermann W, Lewis SL, Hagen S (2011) Benchmark map of forest carbon stocks in tropical regions across three continents. Proc Natl Acad Sci 108:9899–9904
Salazar A, Lennon JT, Dukes JS (2019) Microbial dormancy improves predictability of soil respiration at the seasonal time scale. Biogeochemistry 144:103–116
Schimel JP, Weintraub MN (2003) The implications of exoenzyme activity on microbial carbon and nitrogen limitation in soil: a theoretical model. Soil Biol Biochem 35:549–563
Schlichting E, Blume H-P (1967) Bodenkundliches Praktikum. Paul Parey Verlag, Hamburg und Berlin
Shahzad T, Chenu C, Genet P, Barot S, Perveen N, Mougin C, Fontaine S (2015) Contribution of exudates, arbuscular mycorrhizal fungi and litter depositions to the rhizosphere priming effect induced by grassland species. Soil Biol Biochem 80:146–155
Siles JA, Díaz-López M, Vera A, Eisenhauer N, Guerra CA, Smith LC, Buscot F, Reitz T, Breitkreuz C, van den Hoogen J, Crowther TW, Orgiazzi A, Kuzyakov Y, Delgado-Baquerizo M, Bastida F (2022) Priming effects in soils across Europe. Glob Change Biol 28:2146–2157. https://doi.org/10.1111/gcb.16062
Sinsabaugh RL, Turner BL, Talbot JM, Waring BG, Powers JS, Kuske CR, Moorhead DL, Follstadt Shah J (2016) Stoichiometry of microbial carbon use efficiency in soils. Ecol Monogr 86(2):172–189
Sitton JD, Story BA (2006) Estimating soil classification via quantitative and qualitative field testing for use in constructing compressed earth blocks. Procedia Eng 145:860–867
Soares M, Rousk J (2019) Microbial growth and carbon use efficiency in soil: links to fungal-bacterial dominance, SOC-quality and stoichiometry. Soil Biol Biochem 131:195–205
Soong JL, Marañon-Jimenez S, Cotrufo MF, Boeckx P, Bodé S, Guenet B, Peñuelas J, Richter A, Stahl C, Verbruggen E, Janssens IA (2018) Soil microbial CNP and respiration responses to organic matter and nutrient additions: evidence from a tropical soil incubation. Soil Biol Biochem 122:141–149
Spearman C (1904) The proof and measurement of association between two things. Am J Psychol 15(1):72–101. https://doi.org/10.2307/1412159
Spohn M (2016) Element cycling as driven by stoichiometric homeostasis of soil microorganisms. Basic Appl Ecol 17(6):471–478
Sullivan PF, Stokes MC, McMillan CK, Weintraub MN (2020) Labile carbon limits late winter microbial activity near Arctic treeline. Nat Commun 11:4024. https://doi.org/10.1038/s41467-020-17790-5
Sundqvist MK, Giesler R, Graae BJ, Wallander H, Fogelberg E, Wardle DA (2011) Interactive effects of vegetation type and elevation on aboveground and belowground properties in a subarctic tundra. Oikos 120(1):128–142
Tate KR, Ross DJ, Feltham CW (1988) A direct extraction method to estimate soil microbial C: effects of experimental variables and some different calibration procedures. Soil Biol Biochem 20:329–333
UNEP, World Conservation Monitoring Centre, world heritage datasheet: Manú National Park (2017). via http://world-heritage-datasheets.unep-wcmc.org/datasheet/output/site/manu-national-park/. Accessed 18 Mar 2021
van der Putten WH, Marcel M, Visser ME (2010) Predicting species distribution and abundance responses to climate change: why it is essential to include biotic interactions across trophic levels. Royal Society 365(1548):1471–2970
VD LUFA Methodenbuch I (1997) 2. Teillfg. VDLUFA-Verlag, Darmstadt ISBN 978-3-941273-13-9
Wang G, Jia Y, Wei L (2015) Effects of environmental and biotic factors on carbon isotopic fractionation during decomposition of soil organic matter. Sci Rep 5:11043. https://doi.org/10.1038/srep11043
Wang X, Tang C, Severi J, Butterly CR, Baldock JA (2016) Rhizosphere priming effect on soil organic carbon decomposition under plant species differing in soil acidification and root exudation. New Phytol 211(3):864–873
Weintraub M, Schimel S (2005) The seasonal dynamics of amino acids and other nutrients in Alaskan arctic tundra soils. Biogeochemistry 73:359–380
Welch BL (1947) The generalization of “Student’s” problem when several different population variances are involved. Biometrika 34(1–2):28–35. https://doi.org/10.1093/biomet/34.1-2.28
Whitaker J, Ostle N, McNamara NP, Nottingham AT, Stott AW, Bardgett RD, Salinas N, Ccahuana AJQ, Meir P (2014) Microbial carbon mineralization in tropical lowland and montane forest soils of Peru. Microbiology 5:72. https://doi.org/10.3389/fmicb.2014.00720
Wilcox BP, Allen BL, Bryant FC (1988) Description and classification of soils of the high-elevation grasslands of central Peru. Geoderma 42(1):79–94
Wilcoxon F (1945) Individual comparisons by ranking methods. Biom Bull 1(6):80–83. https://doi.org/10.2307/3001968
Wild B, Schnecker J, Alves RJ, Barsukov P, Barta J, Capek P, Gentsch N, Gittel A, Guggenberger G, Lashchinskiy N, Watzka M, Zrazhevskaya G, Richter A (2014) Input of easily available organic C and N stimulates microbial decomposition of soil organic matter in arctic permafrost soil. Soil Biol Biochem 75:143–151
Wild B, Li J, Pihlblad J, Bengtson P, Rütting T (2019) Decoupling of priming and microbial N mining during a short-term soil incubation. Soil Biol Biochem 129:71–79
Wookey PA, Aerts R, Bardgett RD, Baptist F, Brathen KA, Cornelissen JH, Gough L, Hartley IP, Hopkins DW, Lavorel S, Shaver GR (2009) Ecosystem feedbacks and cascade processes: understanding their role in the responses of Arctic and alpine ecosystems to environmental change. Glob Change Biol 15:1153–1172
Yang S, Cammeraat ELH, Jansen B, Den Haan M, van Loon E, Rechaarte J (2018) Soil organic carbon stocks controlled by lithology and soil depth in a Peruvian alpine grassland of the Andes. CATENA 171:11–21
Zhang K, Ni Y, Liu X, Chu H (2020) Microbes changed their carbon use strategy to regulate the priming effect in an 11-year nitrogen addition experiment in grassland. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2020.138645
Zimmermann M, Meir P, Silman RM, Fedders A, Gibbon A, Malhi Y, Urrego DH, Bush MB, Feeley KJ, Garcia KC, Dargie GC, Farfan WR, Goetz BP, Johnson WT, Kline KM, Modi AT, Rurau NMQ, Staudt BT, Zamora F (2010) No differences in soil carbon stocks across the tree line in the Peruvian Andes. Ecosystems 13(1):62–74
Acknowledgments
JM was supported by studentship (NE/L002434/1) granted by the UK Natural Environment Research Council (NERC). The authors thank the teams in Cusco and Abisko for their help with fieldwork and Annette Ryan, Kelly Mason and Rebecca Rowe for their advice in the lab.
Funding
JM was supported by studentship (NE/L002434/1) granted by the UK Natural Environment Research Council (NERC).
Author information
Authors and Affiliations
Contributions
JM, IPH and JW designed the experiment; JM, AJCQ and KMB conducted fieldwork; JM, RCB and LR conducted the experiment; JM and CvM analysed the data; all authors contributed to and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no financial or non-financial conflicts to disclose.
Additional information
Responsible Editor: Stephen D. Sebestyen.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Michel, J., Hartley, I.P., Buckeridge, K.M. et al. Preferential substrate use decreases priming effects in contrasting treeline soils. Biogeochemistry 162, 141–161 (2023). https://doi.org/10.1007/s10533-022-00996-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-022-00996-8