Abstract
Ocean acidification is one of the most dramatic effects of the massive atmospheric release of anthropogenic carbon dioxide (CO2) that has occurred since the Industrial Revolution, although its effects on marine ecosystems are not well understood. Submarine volcanic hydrothermal fields have geochemical conditions that provide opportunities to characterise the effects of elevated levels of seawater CO2 on marine life in the field. Here, we review the geochemical aspects of shallow marine CO2-rich seeps worldwide, focusing on both gas composition and water chemistry. We then describe the geochemical effects of volcanic CO2 seepage on the overlying seawater column. We also present new geochemical data and the first synthesis of marine biological community changes from one of the best-studied marine CO2 seep sites in the world (off Vulcano Island, Sicily). In areas of intense bubbling, extremely high levels of pCO2 (> 10,000 μatm) result in low seawater pH (< 6) and undersaturation of aragonite and calcite in an area devoid of calcified organisms such as shelled molluscs and hard corals. Around 100–400 m away from the Vulcano seeps the geochemistry of the seawater becomes analogous to future ocean acidification conditions with dissolved carbon dioxide levels falling from 900 to 420 μatm as seawater pH rises from 7.6 to 8.0. Calcified species such as coralline algae and sea urchins fare increasingly well as sessile communities shift from domination by a few resilient species (such as uncalcified algae and polychaetes) to a diverse and complex community (including abundant calcified algae and sea urchins) as the seawater returns to ambient levels of CO2. Laboratory advances in our understanding of species sensitivity to high CO2 and low pH seawater, reveal how marine organisms react to simulated ocean acidification conditions (e.g., using energetic trade-offs for calcification, reproduction, growth and survival). Research at volcanic marine seeps, such as those off Vulcano, highlight consistent ecosystem responses to rising levels of seawater CO2, with the simplification of food webs, losses in functional diversity and reduced provisioning of goods and services for humans.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rising atmospheric carbon dioxide levels, from fossil fuel combustion and other human activities, are increasing seawater CO2 levels and reducing the carbonate saturation of estuarine, coastal, and surface open-ocean waters (IPCC 2019; Doney et al. 2020). This has globally expanded the volume of seawater that is corrosive to carbonate (Tittensor et al. 2009) and first came to world attention when ocean acidification caused mass oyster spat mortality, threatening a $270 million/year aquaculture industry in Washington State, USA (Ekstrom et al. 2015). Field manipulations of seawater CO2 have shown that ocean acidification causes carbonate saturation state to fall below levels suitable for globally important coral reefs (Albright et al. 2018) and laboratory studies show that acidification can reduce the nutritional value of seafood as it lowers caloric content and levels of protein, lipid and carbohydrate (Lemasson et al. 2019). Thus acidification puts at risk many of the valuable ecosystem services that the ocean provides to society, such as fisheries, aquaculture, and shoreline protection (Hall-Spencer and Harvey 2019; Doney et al. 2020).
Although the chemistry of ocean acidification (OA) is well understood, there is uncertainty about the ecosystem effects of these changes. Laboratory experiments show that OA may affect all marine life, for example through changes in gene expression, physiology, reproduction and behaviour (IPCC 2019). However, it is difficult to scale-up from these results as most laboratory studies address only the effect of one or two parameters on a single organism, since multiparameter studies using multiple species are logistically challenging (Riebesell and Gattuso 2015). In the past 10 years, work using natural gradients in pCO2 has shown the long-term effects of OA on ecosystem properties, functions and services. Many studies have been conducted at shallow submarine hydrothermal vents around active volcanoes (here referred to as hydrothermal CO2 seeps), where large amounts of CO2 are injected into seawater (Fig. 1; Hall-Spencer et al. 2008). These shallow (< 200 m) hydrothermal CO2 seeps (Tarasov et al. 2005; Dando 2010; González-Delgado and Hernández 2018) provide natural analogues to study the impact of OA on ecosystems. Volcanic CO2 seeps also help further our understanding of the subsurface hydrothermal structure of coastal volcanoes, where a significant fraction of the hydrothermal output can occur offshore (Italiano and Nuccio 1991; Pichler et al. 1999a; Di Napoli et al. 2016). From a geochemical viewpoint, these sites allow studies of the dispersion patterns of seep-carried CO2 and water-soluble chemicals in seawater (Boatta et al. 2013; Price and Giovannelli 2017; Pichler et al. 2019).
Here, we review the geochemistry of hydrothermal CO2 seeps and use one of the best-known sites (off Vulcano Island, Sicily) as a case study. This expands on (and complements) recent excellent reviews on the topic (Dando 2010; Price and Giovannelli 2017; González-Delgado and Hernández 2018) by (i) combining available gas and aqueous compositional data for 56 seeps to illustrate quantitatively (in a set of key plots) the processes that govern and control their chemistry; (ii) providing a combination of new geochemical and biological data from Vulcano, including a demonstration on the potential of novel geochemical techniques to study seeps; (iii) presenting a novel and systematic analysis of biological data on how the abundances of higher taxa change at Vulcano in response to OA. Doing so, we present recent advances in biological research when using volcanic CO2 seeps to assess the potential effects of OA and discuss ecosystem responses, describing which organisms survive well in acidified conditions and which do not. We end by presenting future research paths to be explored.
Marine CO2 seeps
Types of submarine CO2 seeps
Carbon dioxide-rich fluid venting on the seafloor is a common geological process. The discovery of vigorous fluid venting at Middle-Oceanic Ridges (MOR) in the 1970s boosted a plethora of studies on both the chemical properties of these ‘black and white smokers’ and on the biological communities leaving therein (Von Damm 1990; Tunnicliffe 1991). Gas venting at MORs is now recognised as a primary pathway of volatile (CO2, noble gases) exchange from the Earth’s mantle to the hydrosphere and atmosphere (Marty et al. 2013). MOR fluids have also been intensively studied because they transport the key chemical ingredients for life (e.g., reduced compounds of the elements C, O, N, S), and are thus potential candidate sites for the origin of life on our planet (Baross and Hoffman 1985). Recent focus has been on these fluids as sources of Fe, Mn, Zn and other micronutrients to the ocean, and on their role in the formation of massive sulphide deposits (Hannington et al. 2005, 2011). However, MOR seeps, alongside with their equivalent in active subduction zones (see de Ronde and Stucker 2015 for a review), typically occur several km deep in the ocean, far from coasts and well below the euphotic zone. They have low biological diversity and a fauna that relies on carbon fixation by chemosynthesis (Tunnicliffe 1991). Consequently, these deep vents are nosuitable proxies for studying the effects of OA on coastal marine biological communities (Dando 2010; González-Delgado and Hernández 2018).
Here, we focus on CO2 seeps in shallow (< 200 m) waters which have marine communities that are more diverse and are not dominated by chemosynthetic organisms (Tarasov et al. 2005; Dando 2010; González-Delgado and Hernández 2018). Shallow high CO2 areas can be produced by a variety of processes, including (González-Delgado and Hernández 2018): (i) karstic groundwater inflow (e.g., Yucatan Peninsula, Gulf of Mexico; Crook et al. 2016); (ii) rainwater and meteoric water inflow into small confined seawater basins, combined with high rate of respiration by living organisms (e.g., Rocas Islands of Palau Oceania; Shamberger et al. 2014); (iii) upwelling of seawater rich in inorganic C (e.g., Kiel Bay in the Baltic Sea; Thomsen et al. 2010); and (iv) discharge at the seafloor of CO2-rich volcanic-hydrothermal fluids. This latter process can lead to both seawater acidification and to (small and confined) temperature increases (Di Napoli et al. 2016). It is on category (iv) above that we focus on below.
Global occurrence of shallow hydrothermal CO2 seeps, and their geological significance
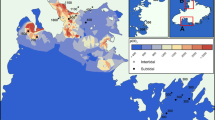
Submarine CO2 seeps are likely to exist around any active coastal or island volcano, or on top of shallow seamounts (Price and Giovannelli 2017). The relatively small number of reports reflects technical challenges in locating and exploring them. Of the 70 reported shallow volcano-related seep locations (Price and Giovannelli 2017), we have identified 56 (Fig. 2) for which chemical and biological information exists (see Table S1, Fig. 2 caption for data provenance). The geographical distribution of these sites, illustrated in the map of Fig. 2, shows clusters in specific areas (e.g., the Mediterranean, Japan-southeast Asia and US). In the field, volcano-related seeps occur as single, gas-rich vents bubbling through the overlying seawater column or, more frequently, as cluster of vents forming sites/fields tens to several hundred square meters in size (Fig. 1). In addition to focused vent emissions, so called “diffuse vents” have also commonly been reported.
Throughout this review, we refer to these volcano-related seeps as hydrothermal CO2 seeps because, geologically speaking, they are due to the interaction between fluids rising from volcanic hydrothermal systems and seawater (Fig. 3). These dynamic environments exhibit large spatial and temporal variations in aqueous fluid and gas chemistry (as well as in their discharge rates), owing to the complex interplay of factors such as local hydrothermal/geological setting, temperature, pressure, pH, fluid-rock interaction extent, and host rock geology. Broadly speaking, submarine hydrothermal systems form as seawater-derived (or meteoric-derived) waters percolate through permeable volcanic rocks, and become heated by dissolving magmatic volatiles rising from deep-seated magma storage zones (Price and Giovannelli 2017) (Fig. 3). As such, hydrothermal systems act as chemical filters, scavenging water-soluble magmatic volatiles to hydrothermal solutions (as Cl−, SO42−, HS−, etc.) and minerals (sulphides, silicates), and converting magmatic volatiles (CO2, CO, SO2) into hydrothermal gases (CH4, H2S) via equilibrium gas–water-rock reactions at reservoir P–T-redox conditions (Giggenbach 1980, 1997; Chiodini and Marini 1998; Fischer and Chiodini 2015) (Fig. 3). At reservoir conditions, hydrothermal fluids are typically two-phase (liquid + reservoir gas) but liquid-dominated (Giggenbach 1997). This aqueous phase is typically a Na–K–Cl brine (Price and Giovannelli 2017) enriched in alkalis and Ca due to extensive leaching of the host rocks (see “Aqueous fluid chemistry” section), and boils to steam as it decompresses upon ascent through geological discontinuities (faults or permeability barriers) (Chiodini and Marini 1998). Upon approach to the near-seafloor, steam and brine mix with cool infiltrating seawater, enriching the residual steam phase in CO2 (that has limited water solubility at hydrothermal conditions) and the residual liquid in seawater components (Mg, Na, K, Cl). Finally, upon discharge, the CO2-rich gases bubble through, and partially dissolve into, seawater, increasing seawater CO2 partial pressure (pCO2) and Dissolved Inorganic Carbon (DIC), and lowering surrounding seawater pH. The C-rich plumes that develop in the water column range considerably in size, as they depend on vent/site/field geometry, fluid (liquid + gas) output rate, temperature (controlling buoyancy) and hydrodynamic parameters (e.g., seawater current) (Fig. 3). The gradients in seawater carbonate chemistry create environments that allow studies into the impact of OA on local ecosystems.
Geological sketch (not to scale) illustrating the mechanisms governing origin and chemistry of CO2 hydrothermal seeps at a costal/insular volcano (see discussion). Red and orange arrows refer to magmatic volatile release from magma and injection into the hydrothermal system, respectively. Light blue and grey arrows identify meteoric recharge and entraining seawater, respectively. The white arrows indicate increasing DIC/pCO2 and Saturation Index (SI)/pH in the seawater column. (Color figure online)
The Baia di Levante hydrothermal CO2 vents
One CO2 seep intensively studied by OA research is the Baia di Levante site in Vulcano Island (Aeolian Islands, Sicily) (Boatta et al. 2013). This small bay, located on the north-eastern margin of the island (Fig. 4), is well known since antiquity for the presence of numerous CO2-rich submarine fumaroles discharging on the seafloor at less than a few meters depth (Italiano and Nuccio 1991; Italiano et al. 1984, 1994; Chiodini et al. 1993, 1995; Capaccioni et al. 2001; Italiano 2009). These fumaroles have compositions typical of hydrothermal steam samples (~ 90 mol% H2O; ~ 90 mol% CO2 and 2 mol% H2S on a dry gas basis; see “Chemical impact of hydrothermal CO2 venting on the seawater column” section), and are thus interpreted as vapours deriving from boiling of hydrothermal brines rising from a shallow geothermal aquifer, identified during exploratory drilling (in 1951–1957) in the Levante Bay beach area (Sommaruga 1984). The geothermal aquifer itself is thought to be fed by magma-derived fluids similar to those feeding the La Fossa Crater further to the south (Chiodini et al. 1993, 1995; Paonita et al. 2013), although some authors (e.g., Tedesco 1995) have argued for a link with the Vulcanello magmatic system instead (Fig. 4). The dissolution of rising H2S-rich hydrothermal steam leads to reduced (Eh as low as − 150 mV) and H2S-rich (166–273 μmol/kg) conditions close to shore at the main submarine vents in the bay (Sedwick and Stüben 1996; Boatta et al. 2013). The high CO2 output (~ 3.6 ton/day of CO2 are discharged by submarine fumaroles into the bay; Inguaggiato et al. 2012) results in acidic conditions (pH as low as ~ 6.5; see below) close to the vents. Also, CO2 seeping at the seafloor leads to strong pH gradients throughout the entire bay, with pH ranging from vent-related values (< 6) to normal seawater levels (8.1) in a few hundreds meters from the vent (see below). These conditions make the Baia di Levante a natural laboratory to characterise the impact of OA on marine ecosystems (Boatta et al. 2013).
Chemistry of hydrothermal CO2 seeps
Gas geochemistry
Collecting total (gas + steam) seep samples is technically difficult in the field. The steam fraction of shallow submarine hydrothermal CO2 seeps is in fact rarely measured (gas analyses are typically expressed on a dry basis, as mol%, ppmv, mmol/mol or μmol/mol of the dry free gas; see Table S2). However, the few data available (e.g., Chiodini et al. 1995) confirm that, similar to their subaerial equivalents (Giggenbach 1980; Chiodini and Marini 1998), submarine hydrothermal seeps are mainly dominated by steam.
On a dry basis, shallow hydrothermal seeps are dominated by CO2. Table S2 lists some free gas analyses taken at several CO2 seeps worldwide (see Fig. 2 for their location). Our compilation is not exhaustive of all shallow submarine vents studied, but includes gas compositional data for all (at least, to the best of our knowledge) those seeps studied so far for their ecology and biology. As such, our compilation is based upon (and is an updated version of) previous cataloguing efforts (Tarasov et al. 2005; Dando 2010; Price and Giovannelli 2017; Gonzáles-Delgado and Hernández 2018; Global CCS institute 2018).
In our inventory (n = 207 gas analyses), the mean CO2 content is 87 ± 20.7 vol%. The range is wide, from 0.4 to 99.6 vol%, but only 14 out of the 207 gas analyses have CO2 < 50%. Where this occurs, the reduced CO2 contents are systemically associated with increasing N2 and O2 contents (Table S2). Thus, the large spread of CO2 contents in our compilation mostly reflects variable extents of magmatic gas dilution by air components (Fig. 5a), either deep in the hydrothermal reservoir or (more likely) during steam migration toward the surface and/or upon discharge on the seafloor. Shallow dilution by air is likely implicated by samples with N2/O2 of ~ 3.7 (the typical air ratio), while deeper (reservoir) air entrainment is perhaps more likely for N2-rich samples with N2/O2 ratios > > 3.7. In hydrothermal reservoirs, atmospheric oxygen is consumed by redox reactions between fluids and host rocks (Giggenbach 1987) and, when this happens, the resulting surface gas emissions typically depart from the pure CO2-air mixing line (green solid line of Fig. 5). In addition to atmospheric O2 consumption, magmatic N2 feeding to the source hydrothermal systems (Pichler et al. 1999a) can also justify the N2-enriched samples. Figure 5a also shows that the Baia di Levante seeps plot at the CO2-rich range (CO2: 94.5 ± 5.9 vol%) of the global population, with only two samples having < 80% CO2 (and > 20% N2).
a Triangular plot of CO2, N2, and O2 contents in hydrothermal CO2 seeps worldwide (data from Table S2). The mixing curve between pure CO2 and air is shown in green; b scatter plot of CO2/CH4 vs. CO2/H2S ratios in hydrothermal CO2 seeps. The red arrows in the red box (top right) illustrate schematically the compositional change resulting from the different processes that govern the global variability in CO2 seep composition. Curves B1–B4 are obtained from iso-enthalpic boiling of four distinct reservoir fluids (black squares) at 150–350 °C down to a separation temperature (grey squares) of 100 °C. Reservoir gas compositions (all in mmol/kg) are: B1: CO2: 500, H2S: 0.1, CH4: 0.1; B2: CO2: 500, H2S: 1, CH4: 0.1; B3: CO2: 500, H2S: 10, CH4: 0.01; B4: CO2: 500, H2S: 10, CH4: 0.1. These compositions are not meant to model any hydrothermal system in particular, but rather to explore the typical compositional range of hydrothermal systems in general
In addition to CO2, N2 and O2, hydrothermal CO2 seeps contain a number of reduced gases at minor (tens of %) to trace (ppmv) levels. H2, CH4 and H2S are the most commonly reported species (Table S2). Deep in the hydrothermal reservoir, these reduced trace components ultimately result from hydrothermal re-equilibration (in either vapour or liquid phase) of the feeding magmatic gas H2O, CO2 and SO2 at reservoir temperature, pressure and redox conditions (expressed by oxygen fugacity, fO2), according to (Giggenbach 1980, 1987):
In the reservoir, hydrothermal H2S (schematically formed by reaction (3)) can also be dissolved into the hydrothermal liquid, or precipitated into newly forming mineral phases, by a variety of reactions, among which:
where FeS2, FeS and F3O4 are hydrothermal minerals pyrite, pyrrhotite and magnetite (native S, sulphates, and dissolved SO42−, can also be formed; Giggenbach 1980). Thus, at least to a first order, the H2, CH4 and H2S contents in the hydrothermal seeps discharged at the seafloor will reflect the T–P–fO2 conditions of their source reservoirs, e.g., they will record to some extent the gas compositions fixed by the T–P–fO2 dependencies of relations (1) to (5), and by the extent of S scrubbing to hydrothermal brines/minerals. Because hydrothermal reservoirs can span a wide range of T–P–fO2conditions (Chiodini and Marini 1998), it is not surprising that the measured H2, CH4 and H2S contents in the CO2 seeps vary so widely in our catalogues, as graphically illustrated by the CO2/CH4 vs. CO2/H2S scatter plot of Fig. 5b. For CH4, part of the spread can also be justified by additional contributions from other sources/processes, including biogenic and thermogenic sources (e.g., low- and high-T organic matter decomposition) and/or abiotic methane formed by reaction other than (2) (e.g., via the Fischer Tropsch reaction) (Chen et al. 2016 and references therein). However, we cannot rule out the possibility that, for such minor components, part of the variability is due to sampling artefacts (e.g., O2 entrainment, and consequent oxidation) and/or analytical/instrumental errors.
An additional complication is related to the contrasting aqueous solubility of CO2, H2, CH4 and H2S at hydrothermal conditions (Giggenbach 1980), because of which these gases will differentially separate into the hydrothermal steam that forms as reservoir fluids rise (from the reservoir) toward the surface and boil (Chiodini and Marini 1998). To illustrate the effect of boiling, four distinct boiling curves (B1-B4) are shown in Fig. 5b calculated for iso-enthalpic boiling of different hypothetical reservoir fluids (with initial CO2, H2S and CH4 contents of 500, 0.1–10 and 0.001–0.1 mmol/kg). These lines are only shown for the sake of illustration, and do not attempt in any case to quantitatively mimic the trends exhibited by natural samples. Yet, the model boiling curves demonstrate that the CO2/CH4 ratio will increase, and the CO2/H2S ratio will decrease, as reservoir fluids cool by iso-enthalpic boiling, from 150–350 °C (the characteristic temperature range of hydrothermal reservoirs; Chiodini ad Marini, 1998) down to a separation temperature of 100° (the seafloor discharge temperature of the majority of the CO2 seeps). The curves also show that, for a given reservoir fluid composition, the ‘reservoir’ gas CO2/CH4 ratio will increase with reservoir temperature (compares curves B1, B2 and B4 in Fig. 5b).
Because of their contrasting aqueous solubilities, CO2, H2, CH4 and H2S can undergo additional fractionation as the rising hydrothermal steam approaches the seafloor, interacts with seawater, and eventually condenses. For example, Caracausi et al. (2005) demonstrated the chemistry of the temporal/spatial variability in the Panarea CO2 seeps in 2002–2003 was strongly linked to selective low-temperature gas dissolution in seawater during steam condensation, perhaps in the vents’ shallow feeding (conduit) systems.
The Baia di Levante CO2 seeps cluster into two distinct areas (Fig. 5b). A first group is characterised by extremely high (> 100,000) CO2/CH4 ratios, and have been interpreted as formed by boiling down to 100 °C of fluids ascending from a ~ 150 °C reservoir (Capaccioni et al. 2001). A second cluster of samples is characterised by lower CO2/CH4 ratios (~ 1000) and somewhat higher (up to > 1000) CO2/H2S ratios, which we ascribe to a combination of (i) some extent of atmospheric dilution (many samples fall on, or nearby of, the air-pure CO2 mixing line), (ii) lower reservoir temperatures (~ 100 °C; Capaccioni et al. 2001), and (iii) higher extents of S scrubbing to hydrothermal minerals (pyrrhotite, according to Capaccioni et al. 2001).
Concern has been raised (see discussion in Gonzáles-Delgado and Hernández 2018) that the presence of reducing compounds (in particular H2S, being toxic for biota) in hydrothermal CO2 seeps may render them unsuitable proxies for the real natural conditions of a future CO2-rich ocean. A look into the Earth’s geological past shows, however, that periods characterised by high atmospheric CO2 (Foster et al. 2017) have not only been associated with global ocean warming, but have also recurrently led to oceanic anoxia, where reducing gas compounds (such as H2S and CH4) do become significant (Jenkyns 2003; Meyer and Kump 2008). For example, in the high-CO2 Mesozoic greenhouse world (Royer et al. 2007), a number of well-documented Oceanic Anoxic Events (OAEs) have been identified (Jenkyns 2010), such as in the early Toarcian (T-OAE; ~ 183 Ma), early Aptian (OAE 1a; ~ 120 Ma), early Albian (OAE 1b; ~ 111 Ma) and Cenomanian-Tutonian (OAE 2; ~ 93 Ma). These OAEs, similarly to that at the Paleozoic–Mesozoic boundary (Wignall and Twitchett 2002), have been caused by rapid perturbation of the atmospheric carbon cycle (Hesselbo et al. 2000; Jenkyns 2010), and have led to biological crises and, in the most extreme cases, to severe mass extinctions (Wignall and Twitchett 1996; Garilli et al. 2015). If, as the geological record suggests, a link exists between swift changes in the C cycle and the establishment of reducing conditions in the ocean, it is possible that the CH4- and H2S-rich nature of hydrothermal CO2 seeps may reinforce their use as proxies for ongoing/future man-made oceanic acidification.
CO2 output
The impact of shallow submarine hydrothermal CO2 seeps on the chemistry of the overlying seawater column (see “Chemical impact of hydrothermal CO2 venting on the seawater column” section) depends on many factors, including the geometry of the fumarolic field, the hydrodynamic properties of the seawater column itself (e.g. seawater current and any vertical stratification in temperature and density), and the CO2 mass output. This latter parameter (the mass of CO2 released by a hydrothermal CO2 vent area/field per unit time) is critical in determining the horizontal and vertical extension of the pCO2 and dissolved inorganic carbon (DIC) anomaly in seawater.
The Panarea hydrothermal field in northern Sicilian Tyrrhenian coast is the only site to our knowledge for which a systematic temporal record of the CO2 output exists. Based on using a direct method, Italiano and Nuccio (1991) estimated a typical CO2 output for this ~ 4 km2 submarine field of ~ 9 × 106 l/day (corresponding to ~ 26–32 tons/day at the 30–100 °C temperature of the vents). This CO2 output is estimated to have increased by more than one order of magnitude (up to ~ 4 × 108 l/day) in correspondence to a violent gas burst event that occurred on November 2002 (Caliro et al. 2004). Di Napoli et al. (2016) used the HydroC™, CONTROS sensor (Fietzek et al. 2014) for real-time measurements of dissolved CO2 at high temporal and spatial resolution in the seawater column above the Secca delle Fumose CO2 seep field, Pozzuoli Bay (Italy). From detailed 3D mapping of pCO2 in seawater, they estimated a CO2 output from the Secca delle Fumose field (~ 0.14 km2) of ~ 50 tons/day (or ~ 0.53 kg/s). This is more than a factor 10 higher that the estimated CO2 output from the Baia di Levante submarine seeps on Vulcano Island (~ 3.6 ton/day; Inguaggiato et al. 2012).
Hydrothermal seeps are very dynamic systems, as the deep geological processes that drive their activity can exhibit large fluctuations in time (Caliro et al. 2004). Obtaining temporal records of CO2 output variations at submarine hydrothermal CO2 seeps is thus a key objective of future studies.
Aqueous fluid chemistry
In addition to gas, saline hot water (highest reported temperature, 135 °C; Price et al. 2015) is typically discharged from the seafloor by shallow hydrothermal CO2 seeps (Price and Giovannelli 2017). These thermal waters can be acidic (Fig. 7) and contain bio-limiting nutrients or toxic elements (Tarasov et al. 2005; Price et al. 2013, 2015; Price and Giovannelli 2017; Pichler et al. 2019; Lu et al. 2020), so geochemical survey work was needed at Vulcano Island to map out areas well suited for ocean acidification studies, away from the influence of factors that would confound the effects of increasing CO2 levels, such as toxic H2S (Boatta et al. 2013).
Hydrothermal seep fluids vary widely in composition (Figs. 6, 7) but the relative abundances of anions (Fig. 6a) and cations (Fig. 6b) is usually similar to that of seawater. Most such seeps are fed by infiltrated seawater (although there are examples of meteoric water-fed systems; Pichler et al. 1999a) with mixing with cold seawater during fluid ascent from a reservoir as well as during seafloor discharge (Price et al. 2015; Price and Giovannelli 2017). Relative to seawater, many seeps are enriched in carbon (Fig. 6a) due to CO2 dissolution sourced by either hydrothermal steam (formed by steam separation from deeper reservoir fluids; Pichler et al. 1999a; cfr 3.1) or magmatic vapours separated by the magmatic source (Caliro et al. 2004). Sulfur is typically depleted relative to seawater due to deposition of hydrothermal sulphates (Alt 1995; German and Von Damm 2003) and sulphides (Hannington et al. 2011) upon hydrothermal infiltration and maturation (Fig. 6a).

Data Sources Milos: Valsami-Jones et al. (2005), Price et al. (2013), Lu et al. (2020); Tatum bay, PNG: Pichler et al. (1999a), Pichler et al. (2019); Vulcano Island: Sedwick and Stuben (1996), Boatta et al. (2013); Panarea: Italiano and Nuccio (1991), Caracausi et al. (2005), Price et al. (2015); Upa-upasina, Dobu, Esa’Ala, PNG, Fabricius et al. (2011); Campi Flegrei: Di Napoli et al. (2016)
Triangular plots for anion (a) and cation (b) contents in some selected, well-studied seeps. Data used to draw the plots are listed in Supplementary Table S3.
Scatter plots of pH vs. selected major (Ma, Na), minor (Si) and trace (Fe, Mn, As) contents in some selected seeps (and in the overlying seawater column). Symbols and data-sources are as in Fig. 6
One notable feature of vent fluids (or, at least, of those poorly affected by near-surface mixing with seawater) is their characteristic Mg depletion (Figs. 6b, 7a), which arises from extensive Mg removal from solutions due to formation of hydrothermal phyllosilicates (German and Von Damm 2003; Tivey 2007). Prior to mixing with seawater, hydrothermal vent fluids are, in the majority of the cases, of alkali-chloride composition (Fig. 6b), as typical of ‘mature’ geothermal waters that have attained full-equilibrium conditions after prolonged fluid-rock interaction with the host rocks at hydrothermal reservoir P–T-conditions (Giggenbach 1988). These fluids exhibit a range of alkalis/Ca ratios (Fig. 6b), reflecting different extents and environments (P–T-conditions) of fluid-mineral interaction from one seep system to another, or even within one single system (Price et al. 2015; Price and Giovannelli 2017).
Vent fluids exhibit a wide range of salt contents, as exemplified for Na in Fig. 7b, implying a multiplicity of sources and processes. A large cluster of seeps plot at Na concentrations slightly lower than seawater, suggesting seawater recharge followed by some extent of Na removal by hydrothermal minerals (e.g., albitization; Tivey 2007) during mineral–fluid interaction. The numerous vents with Na contents plotting well above (e.g., a subset of vents samples form Milos and Panarea) or below (e.g., Tatum bay) seawater (Fig. 7b), point to additional processes, however, and indicate in particular the recurrence of phase separation processes (Butterfield etal. 1990; Von Damm et al. 2003; Foustoukos and Seyfried 2007). Supercritical (above critical point) phase separation (Hedenquist and Lowenstern 1994) deep in the Panarea hydrothermal system has for example being invoked (Price et al. 2015) to explain the formation of saline brines (Na > 600 mM) vented by some seeps (Fig. 7b); while low salinity vent fluids (Na < 400 mM) have recurrently been explained (Pichler et al. 1999a; Price et al. 2013, 2015) by near-surface condensation of hydrothermal steam, formed by sub-critical phase separation (boiling) at depth.
Prolonged high-temperature interactions with reservoir rocks enrich hydrothermal fluids in a suite of minor elements such as Si (Fig. 7c), alkalis (Li, Rb, Cs) and B (Giggenbach 1997). Some of these elements (e.g., Si, B) exhibit conservative behaviour (e.g., they remain in dissolved forms during mixing of seep waters with seawater, and cooling; Fig. 7c), and can thus serve to quantify the extent of seawater entrainment (the mixing proportions of hydrothermal and marine components in the seeps; Italiano and Nuccio 1991; Pichler et al. 1999a, 2019; Caracausi et al. 2005), and to map dispersion and dilution of seep-derived elements into the seawater column (Pichler et al. 1999a, 2019).
The hot, reducing conditions of hydrothermal systems mobilise trace elements (e.g., Fe, Mn, Cu, Pb, Zn, Cd, As, and many others) by leaching from rocks, leading to orders of magnitude enrichment in seeps relative to seawater (see Tarasov et al. 2005) (Fig. 7d–f). Trace elements play a wide range of possible roles in (and effects on) living organisms, from essential/bio-limiting to harmful/toxic (Stumm and Morgan 1995; Rainbow 2002). As such, care is needed when using these systems as natural proxies or studying OA effects on marine organisms. Trace element contents in seawater surrounding hydrothermal seeps have been measured in several cases, including at Vulcano Island, the main focus of this paper (Boatta et al. 2013; Brinkman 2014; Donnarumma et al. 2019; Pichler et al. 2019; Zitoun et al. 2020). Bio-availability of trace-elements depends on metal speciation (Rainbow 2002; Vizzini et al. 2013; Mishra et al. 2020) and so seeps provide areas in which to assess the effects of ocean acidification on trace element toxicity. One key aspect to address is to assess which metals precipitate to minerals upon contact with cold seawater (e.g., Pichler et al. 1999b; Becke et al. 2009; Price and Pichler 2005; Price et al. 2013) and which dissolve and disperse to potentially have wider-scale consequences on ecosystems.
The trace-element vs. pH trends illustrated in Fig. 7d–f offer some useful (though preliminary) clues. The Mn vs. pH plot of Fig. 7e, for example, shows a relatively uniform trend of increasing Mn concentrations (of up to 7 orders of magnitude above seawater) at increasingly acidic (pHs of 4–7) seep conditions. This points to relatively wide Mn mobility over a range of aqueous environments (Lupton et al. 2011). In contrast, Fe (Fig. 7d) and As (Fig. 7f) show more complex behaviours when plotted vs. pH. Although we cannot rule out these trends partially reflect unsuccessful sampling of pure hydrothermal end-members, we consider more likely that the observed contrast between Mn and Fe/As behaviours imply non-conservative behaviour and scavenging of the latter elements to mineral phases (e.g., oxy-hydroxides and sulphides; e.g., Pichler and Veizer 1999; Pichler et al. 1999a, b; Becke et al. 2009; Price and Pichler 2005; Price et al. 2013) upon mixing/cooling in the upflow zone (especially at pH < 5.5 and 7; Fig. 7). Extensive work on As, in particular, has shown that while this element can be extremely enriched in seeps (Pichler et al. 1999a, b; Price et al. 2013), only moderate enrichments in the overlying seawater column (relative to reference seawater) are produced owing to extensive precipitation of As-rich oxy-hydroxides upon seep discharge (Pichler and Veizer 1999). This fact, combined with lack of visible impacts on coral communities, led Pichler et al. (2019) to conclude that the high As load transported by those seeps did not result in major biological consequences. This topic has been examined in most detail with regard to seagrasses, since the question arose as to why they thrive at some CO2 seeps but not others. At some seeps seagrasses are abundant where CO2 bubbles up through the seabed, and the plants are thought to benefit from the increased availability of Dissolved Inorganic Carbon for photosynthesis and a decreased cover of epiphytic coralline algae on their leaves (Martin et al. 2008; Russell et al. 2013). However seagrasses do badly close to some CO2 seeps due to H2S and/or metal toxicity (Vizzini et al. 2013). At Vulcano and other Mediterranean seeps areas with bioavailable toxins can be avoided by moving away from the seeps to where levels of seawater CO2 are below 1000 µatm (Mishra et al. 2020), although bioavailable species can occasionally reach toxic thresholds in these more distal environments, too. The conclusion is that, although geochemical surveys of seep sites are warranted, trace element enrichment in shallow hydrothermal CO2 vents should not prejudice their use as natural proxies for OA studies. However, as seeps are very dynamic multiple stressor environments, areas suitable for OA studies must be carefully selected using comprehensive (gaseous, major, minor and trace aqueous species) chemical characterization.
Chemical impact of hydrothermal CO2 venting on the seawater column
pCO2 distribution in seawater
Upon exiting the seafloor, CO2 bubbles vented from the seeps dissolve into seawater (Fig. 1), via reactions:
CO2 can dissolve either partially or totally in the seawater column, depending on seep discharge rate (CO2 output; cfr. 3.2) and vent emission depth. If the CO2 output is high enough, and the seawater column only shallow, part of the gas can freely escape at the seawater–air interface, leading to gas bubbling at surface (Fig. 1). In both cases, the ultimate effect is to raise seawater Total Dissolved Inorganic Carbon (TDIC) and CO2 partial pressure (pCO2).
Traditionally, seawater pCO2 values in the surroundings of hydrothermal CO2 seeps have been determined by numerically solving dissolved C speciation from co-measured TDIC and pH. Results obtained at the different CO2 seeps worldwide, illustrated in Fig. 8a, demonstrate a transition from ‘normal seawater’ pCO2 (~ 400 μatm) in background (control) sites far from the vents, to circa ~ 1000 μatm at “intermediate” (meters to tens on meters) distance from the vents, to exceptionally high pCO2s (≥ 10,000 μatm) in near-vent conditions. The most extreme pCO2 conditions (up to 52,000 μatm) have been observed offshore Ischia volcano, in Italy (Hall-Spencer et al. 2008). It is along pCO2 gradients of 300–1000 μatm that biological research focuses in the attempt to characterise the response of biological communities to enhanced seawater CO2 levels (Agostini et al. 2018; see “Biological experiments at volcanic CO2 seeps as analogues of ocean acidification” section).
Scatter plots illustrating seawater carbon chemistry at hydrothermal CO2 seeps worldwide (data from Table S4). a pH vs. pCO2; b pH vs. dissolved C species; c pH vs. calcite Saturation Index (SI); d pH vs. aragonite Saturation Index (SI). pCO2, dissolved C speciation and SIs are equilibrium values calculated from measured pH and Total Dissolved Inorganic Carbon (TDIC)
The advent of novel, portable instruments for real-time measurement of dissolved CO2 in seawater is now paving the way to mapping seawater pCO2 distribution around hydrothermal seeps at much finer spatial resolution (e.g., Di Napoli et al. 2016). In our Baia di Levante example, we used a HydroC™ pCO2 sensor (from CONTROS Systems and Solutions, Fietzek et al. 2014) and a multi-parameter probe (Ocean Seven 303 CTD, IDRONAUT) to map the pCO2 in the bay (Fig. 9a, b). The pCO2s obtained from both sensors (HydroC™ and Ocean Seven 303) matched well those derived from traditional techniques (Capasso and Inguaggiato 1998) that use directly sampled seawater samples (see Fig. 9c), providing further validation for these new generations of instruments (Fietzek et al. 2014).
a A map of seawater pCO2 distribution in the Baia di Levante bay. The map was generated from in-situ real-time measurements of dissolved CO2, obtained using a HydroC™ sensor (for CO2 < 6000 μatm) and an Ocean Seven 303 IDRONAUT CTD (for CO2 > 6000 μatm). Measurements were obtained using a small boat and proofing along the track shown in Fig. 4 (bottom, right). The use of the HydroC™ and IDRONAUT CTD sensors to measurement of dissolved CO2 in seawater was initially validated against results from conventional direct sampling, as shown in (c). Water samples for sensor comparison were collected in sites indicated by white circles; b a detail of (a). Stars identify the position of the main degassing vents (where gas bubbling at the free seawater surface was also concentrated); c comparison between pCO2 values measured by the HydroC™ sensor (orange diamonds) and the Ocean Seven 303 IDRONAUT CTD (green diamonds) (both on the y-axis) and those measured with the direct sampling technique of Capasso and Inguaggiato (1998) method (x-axis). The dashed line represents the 1.1 line (e.g., it identifies perfect match between instrumental and direct sampling derived pCO2s). The best-fit regression lines are respectively: y = 0.99x-65, R2 = 0.93 (for direct vs. HydroC™ datasets) and y = 0.87x-27, R2 = 0.99 (for direct vs. IDRONAUT CTD datasets). Overall, our test shows that the HydroC sensor pCO2 results are well consistent with those derived in laboratory on the directly sampled seawater samples (method of Capasso and Inguaggiato 1998). This agreement provides mutual validation for the 3 techniques. (Color figure online)
Here, we used a small boat to profile the bay along the track shown in Fig. 4, while our sensor kit was continuously acquiring data (at 1 Hz rate). From this, the lateral variability of seawater pCO2 at a constant depth (~ 0.5 m) was obtained, which is ultimately illustrated by the map of Fig. 9. The map demonstrates the ability of these novel sensor kits to finely resolve (in real-time) dissolved CO2 in seawater around seeps with much improved spatial resolution than possible with traditional methods (direct sampling). We recommend increased use of these sensors in future OA and seabed carbon capture and storage studies.
pH and ocean acidification
The dissolution of CO2 in seawater around seeps has several environmental consequences. The dissociation of the carbonic acid (H2CO3(aq); see relation (7)) lowers the pH (Fig. 8a), alters the normal seawater dissolved carbon speciation (Fig. 8b), and lowers the seawater saturation state of calcite and aragonite (Fig. 8c, d). At intermediate conditions, meters to tens of meters from the seeps, seawater acidity is typically up to 0.2–1 log units lower (pH of 8 to 7) than ambient seawater (Fig. 8a), dissolved CO2(aq) concentrations increases at the expense of the carbonate ion (CO32−(aq)) (the two species attain similar abundances at pH ~ 7.5, while CO2(aq) prevails over CO32−(aq) at pH < 7.5; Fig. 8b), and the calcite/aragonites Saturation Indexes (SIs) drop, from ~ 4–6 to ~ 1–2 (Fig. 8b, c). In the most extreme conditions (in the near-vent environment), where pCO2 exceeds 10,000 μatm, the pH becomes acidic (6.5 to 6; Fig. 8a) and CO32−(aq) concentrations are largely suppressed (Fig. 8b), ultimately unstabilising carbonate minerals (Fig. 8c, d). The same transition, from ‘normal seawater’ to ‘acidified seawater’ conditions can be captured by independent pCO2 and pH measurements while approaching the active seeps, as in our Baia di Levante example (Fig. 10).
Seawater pH vs. pCO2 scatter plotat Baia di Levante (Vulcano Island). pH was measured with the Ocean Seven 303 IDRONAUT CTD, while pCO2 was real-time in-situ determined via either the HydroC™ sensor (for pCO2 < 6000 μatm) or the Ocean Seven 303 IDRONAUT CTD (for pCO2 > 6000 μatm). Measurements were taken at constant seawater depth at a constant depth of ~ 0.5 m along the measurement grid shown in Fig. 4. We categorize samples in near-vent sites (pH < 7), intermediate sites (7 < pH < 8) and near-ambient sites (pH > 8). These three categories correspond to distances from the vent areas of < 10 m, 10–100 m and > 100 m, respectively
Biological experiments at volcanic CO2 seeps as analogues of ocean acidification
In addition to their global significance in geological CO2 flux, many submarine seeps provide opportunities to improve predictions about the effects of OA on the marine biota and ecosystem structure and function. Such seeps allow assessments of the effects of high CO2 levels and lowered seawater pH on spatial and temporal scales sufficiently large to set up experiments to address responses from individual organisms to ecosystems (Gaylord et al. 2015; Hall-Spencer and Harvey 2019). Most research using seep sites to assesses the biological effects of OA have avoided localised areas with raised temperature, anoxia and/or toxic metals (Boatta et al. 2013) (Fig. 9), although such sites provide ample opportunities to assess the combined effects of multiple stressors. In this regard, a recent attempt was made along the pCO2/pH gradient off Vulcano Island (Southern Italy), combining the naturally occurring carbonate chemistry gradient with an in-situ manipulation of temperature conditions and assessing how these environmental stressors affect the fitness of a reef building species. Under an end of the century scenario of both stress factors (~ 1000 µatm and + 2 °C) translaplanted vermetid reefs exhibited compromised oxygen consumption and bio-mineralization processes leading to impaired development and recruitment (Alessi et al. 2019).
Two types of experiments are generally employed to investigate the effects of OA at CO2 seeps: observational and transplantation experiments. In-situ observational experiments assess the species responses along natural pH gradients, generally moving progressively away from the most intense CO2 bubbling site to ambient CO2 sites both in intertidal and subtidal zones (e.g. Agostini et al. 2018). Such experiments usually focus on sessile benthic species which are chronically exposed to the pCO2 and pH gradient moving away from the seep site (Agostini et al. 2018). This approach has also highlighted mechanisms underpinning evolutionary responses of calcifying species in past OA events (Garilli et al. 2015).
In-situ observational studies often precede experiments involving field transplantations where researchers, in the attempt to highlight cause-effect mechanisms, compare responses of single species or entire assemblages and communities to both short- and long-term acidified and natural control conditions (Camp et al. 2018). For practical reasons, most experiments at CO2 seeps have focused on low motility benthic stages of organisms, such as microbes, macroalgae, anemones, corals, sessile polychaetes, bryozoans, or bivalve and gastropod molluscs (e.g. Goffredo et al. 2014) rather than planktonic, neustonic or highly mobile organisms such as phytoplankton, cephalopods or fish.
Biological studies at CO2 seeps include the experimental investigation of processes such as calcification/dissolution, growth, survival, settlement, recruitment, reproduction, metabolic performance, behaviour, species interactions and community shifts in relation to spatio-temporal changes in pH, pCO2 and saturation levels of aragonite and calcite (Camp et al. 2018). In some instances, CO2 seep research has provided evidence of direct physiological impacts on individuals living along CO2 gradients, causing impaired growth and reproduction, and hence affecting the population level under elevated pCO2 conditions (Harvey et al. 2016). Other experiments document shifts in habitat-forming species and consequent reshuffling of species at community-level or imbalanced function at ecosystem-level (e.g., Vizzini et al. 2017; Milazzo et al. 2019). Despite the obvious caution needed to avoid confounding factors, biological experiments carried out at CO2 seeps currently provide a powerful tool to assess future ocean conditions, especially for documenting which organisms and processes are most resilient to OA and to assess potential risks for marine populations, communities and ecosystems.
Winners and losers at natural analogues of OA: the Baia di Levante case study
How organisms will respond to OA might depend on energetic trade-offs between the physiological costs of maintaining ion regulation and acid base status and other energetically demanding processes, such as growth, calcification, reproduction, and activity. These determine the life-history traits and therefore the performance and fitness of the organisms. Such responses may be limited not only by a species’ physiological plasticity but also by energy availability in the environment. Despite recent advances in understanding the energetic trade-offs that determine species sensitivity to OA in the laboratory, CO2 seep studies are only starting to reveal how these trade-offs can be affected under natural conditions where species are subjected to additional biotic and abiotic processes that can modify interactions and energy availability (Garilli et al. 2015; Turner et al. 2015).
Meta-analyses of laboratory responses to OA shows that sensitivity varies among life stages, species and broader taxonomic groups (Hendriks et al. 2010; Kroeker et al. 2010, 2013; Harvey et al. 2013; Wittmann and Pörtner 2013; Cattano et al., 2018), although it is difficult to know how this range of responses plays out in the real world without empirical data from natural gradients in OA conditions (Rastrick et al. 2018). Here, we consider the culmination of complex responses of organisms to OA by assessing changes in the abundances of organisms in-situ using effect size analysis (Hedges et al. 1999) along gradients of OA off Vulcano Island (Aeolian Islands, Sicily) (Fig. 11), one of the most intensively used natural laboratories for OA research.
Effect sizes (± 95% CI) on abundances of different key taxa at Baia di Levante (Vulcano Island, Italy). For each response variable, the response ratio (RR) was calculated with Extreme, Intermediate and near Ambient CO2 conditions as numerators (i.e., the experimental condition) and the Control condition as the denominator. Empty diamonds represent null effects, while filled ones represent positive or negative significant effects. The response of each group to different pH conditions was analysed using %cover for Cyanobacteria, Diatoms, Coccolitophores, Filamentous turf algae, Crustose coralline algae, Fleshy algae, shoot density for Seagrasses, and density for Anemones, Bryozoans, Gastropods, Bivalves, Isopods, Amphipods, Decapods, Ostracods, Tanaids, Serpulids, other Polychaetes, Sea Urchins and Fish (as MaxN relative abundance). Data from: Suggett et al. (2012), Calosi et al. (2013), Johnson et al. (2013), Ziveri et al. (2014), Apostolaki et al., (2014), Nagelkerken et al. (2015), Harvey et al. (2016); Vizzini et al. (2017), Milazzo et al. (2019), Mishra et al. (2020); Turco G. unpublished data. Images from https://notendur.hi.is/~salvor/myndvinnsla/inkscape/IAN%20Symbol%20Libraries%205.1%20SVG/
To make comparisons at different pCO2 levels along the gradient we considered a CO2 range (ΔCO2) as the difference between the CO2 concentration in elevated, intermediate, near-ambient and the control CO2 condition, that resulted as extreme (ΔCO2 ≥ 750 µatm), intermediate (750 > ΔCO2 > 350 µatm) and moderate (ΔCO2 ≤ 350 µatm) OA scenarios (Fig. 11).
Our analyses suggest that primary producers like benthic biofilm (cyanobacteria and diatoms), filamentous turf and fleshy algae showed similar magnitude of mean increases in abundance in OA conditions (Fig. 11), as found by Harvey et al. (2019) at Japanese CO2 seeps. The abundance of crustose coralline algae was not affected under OA, and bivalve abundances increased in extreme low pH/high CO2 conditions, which are surprising results given that coralline algae and bivalves have been shown to be adversely affected by OA at volcanic seeps in Papua New Guinea and Japan (Fabricius et al. 2015; Agostini et al. 2018) and the Azores (Martins et al. 2020). However, the proliferation of a few hardy taxa and the demise of many others is a common feature in OA research and a reduction in diversity (rather than abundance) can impair ecosystem services (Hall-Spencer and Harvey 2019). Other calcified taxa showed consistent reductions in abundance in the extreme OA scenario, which were similar among coccolithophores, gastropods, bryozoans and serpulid worms. Our evidence that marine taxa potentially vulnerable to low pH conditions may in some instances thrive under OA is not new, as local environmental conditions like nutrient availability (Connell et al. 2017) or exposure over multiple generations (Cornwall et al. 2020), may help some organisms to thrive (Zitoun et al. 2020).
Consistent increases in abundance in OA conditions were detected for anemones, isopods, amphipods, and other polychaetes at all lower pH/elevated pCO2 levels considered. No effects of acidification were detected on fish abundances (Fig. 11). Our analyses reveal that the magnitude of responses to OA in the field varies among examined groups, suggesting variation in sensitivity when multi-species assemblages are considered with this having important implications for ecosystem responses under OA conditions.
If we look at species diversity, most algal species are resilient to OA, with only around a 5% loss in species diversity at levels projected under the IPCC RCP 8.5 (Brodie et al. 2014). Increased carbon availability stimulates primary production. This can increase the standing stock of kelps and seagrasses (Russell et al. 2013; Linares et al. 2015; Cornwall et al. 2017) although microalgal biofilm and weedy small turf algae dominate at high CO2 levels in exposed conditions (Agostini et al. 2018; Connell et al. 2018). Shifts in algal community composition greatly alter habitat characteristics (Brodie et al. 2014; Enochs et al. 2015) with this having major implications on associated invertebrate and vertebrate fauna (Fabricius et al. 2014; Cattano et al. 2020).
At tropical, sub-tropical and temperate seeps there is a shift to less diverse coastal ecosystems as pCO2 levels increase. There is around a 30% fall in animal biodiversity as average pH declines from 8.1 to 7.8 (Fabricius et al. 2011; Agostini et al. 2018; Foo et al. 2018). This is due to direct and indirect effects, such increased metabolic costs of coping with hypercapnia or increased susceptibility to predation (Sunday et al. 2017). A diverse range of invertebrates build reefs; such as sponges, corals, serpulids, vermetids, oysters, mussels and bryozoans. Reefs are degraded along gradients of decreasing carbonate saturation, due to increased metabolic costs of calcification, chemical dissolution and enhanced bioerosion.
Opportunistic organisms can more easily adapt to acidification (Kroeker et al. 2011; Brown et al. 2018). In the tropics, some corals are able to grow well in acidified conditions, but the habitats they form lack complexity and the reef itself is eroded (Fabricius et al. 2011). These degraded reefs have more fleshy macroalgae, less calcified algae and tend to have fewer invertebrates. The dual effects of increased CO2 and decreased carbonate alter trophic interactions in both sub-tropical and temperate coastal systems. Reductions in the abundance and size of calcareous herbivores contribute to the overgrowth of weedy turf algae and a simplification of food webs, with losses in functional diversity (Vizzini et al. 2017; Teixidó et al. 2018). In this regard, marine ecosystem services depend on what basic biotic functions are maintained (Connell et al. 2018), which ecosystem engineers and keystone species are retained (Sunday et al. 2017), and whether the spread of nuisance species is avoided (Hall-Spencer and Allen 2015).
Ecosystem effects of OA at CO2 seeps
In the past 10 years, experimental work using natural gradients in pCO2/pH has increased worldwide. The ecosystem effects of acidification were first described on a range of coastal Mediterranean habitats off the Castello Aragonese in Ischia Island (Italy). In this shallow seep site, CO2 bubbling up through the seabed gradually lowers the pH of the water column from present day ambient pH 8.2, through near future pH 7.9, down to worse case predictions of pH 7.4 in the immediate vicinity of seeps (Hall-Spencer et al. 2008). Along these pCO2 and pH gradients, rocky shore communities with abundant calcareous organisms shift to communities lacking sea urchins, scleractinian corals and vermetid molluscs and with significant reductions in coralline algae. This approach has provided an ecosystem-scale validation of laboratory-based predictions that important groups of organisms are susceptible to elevated levels of pCO2 in the ocean.
Since then, evidence from CO2 seeps including coral (Fabricius et al. 2011, 2014; Inoue et al. 2013; Enochs et al. 2015; Agostini et al. 2018) and other calcifying reefs (Milazzo et al. 2014; Linares et al. 2015; Alessi et al. 2019), seagrasses (Garrard et al. 2014; Nagelkerken et al. 2015), and algal dominated habitats in temperate zones (Porzio et al. 2011) has notably increased, showing community structure alterations in response to OA (Fig. 12).
Evidence of community shifts from ambient to elevated CO2 levels off a seep site in Papua New Guinea. In ambient locations highly complex and diversified coral reef species which shift into low profile corals and fleshy algae in elevated CO2 conditions close to the bubbling site. Photo credit: Marco Milazzo
A reduction in community complexity is expected following prolonged exposure to OA (e.g. Kroeker et al. 2011; Sunday et al. 2017), primarily due to the loss of CO2-sensitive species (e.g. calcifying habitat-forming species) and an increase in primary producers (e.g. fleshy algae and turf-forming algae) boosted by elevated CO2 conditions (Connell et al. 2018). This shows the dual role of CO2 as a stressor and a resource for marine ecosystems. Biotic homogenization occurs under OA, with systems at low disturbance (i.e. ambient CO2 and pH) hosting specialist species with high complementarity and high ecosystem function, while highly disturbed ecosystems (elevated CO2 and low pH) favoring generalist species and homogenized communities with low function.
Vermetid reef communities transplanted for one year along CO2 gradients off Vulcano Island, revealed dominance shifts from reef- to non reef-forming habitats (Milazzo et al. 2019). These shifts led to changes in composition, structure and functional diversity of the associated benthic assemblages. Interestingly, community properties (i.e. species richness and abundance) nonlinearly responded to OA conditions, high-order consumers (e.g. carnivores) failed to compensate, and competitive release of minor herbivores occurred under elevated CO2 levels (Milazzo et al. 2019). Coastal community assemblages often have low functional redundancy (Micheli and Halpern 2005), meaning that CO2-tolerant species performing a similar functional role may not exist. This suggests the likelihood for the loss of functional traits and groups (e.g. reef-forming species and carnivores) (Sunday et al. 2017; Vizzini et al. 2017; Teixidó et al. 2018), while highlighting the potential for opportunistic species to become more generalist and widen their ecological niche.
Shallow biogenic reefs are particularly sensitive to OA, the degradation of these habitats results in less coastal protection and less habitat provisioning for biodiversity and fisheries. Live coral cover on tropical reefs has nearly halved in the past 150 years, and the decline dramatically has accelerated over the past 2–3 decades due to increased water temperature and OA interacting with and further exacerbating other drivers of loss. Natural gradients in seawater CO2 show that risks to marine goods and services amplify with increasing carbon emissions. When combined with rising temperatures, sea level rise and increasing extreme events, OA further threatens the goods and services provided by coastal ecosystems. This is particularly important for those people and countries that are heavily reliant on marine resources for protection, nutrition, employment and tourism (Lam et al. 2019).
Summary
We have reviewed existing knowledge on the geochemistry and ecology of submarine volcanic CO2 seeps, especially focusing on the lessons these studies bring for predicting the potential impact of OA under a growing atmospheric CO2 planet. We have shown that volcanic seeps are CO2-dominated, but also exhibit a compositional diversity that reflects the complex processes (boiling, mixing and condensation) happening in the subsurface. In addition to CO2, volcanic seeps are rich in reducing species (H2S, CH4, and H2) whose ecological impacts and significance for future oceanic trends is currently poorly understood. In addition to the gas phase, volcanic seeps typically release a saline aqueous phase (brine) rich in a plethora of trace elements of potentially significant biological importance. In the last decade, there has been a clear call for addressing the important effects of OA on high-level systems of life organization (i.e. biological communities and ecosystems), specifically when making predictions about the impacts of ongoing environmental change. We have shown recent advances in ecological research when using volcanic CO2 seeps as natural analogues for addressing ecosystem effects and winner and loser species under OA. Indeed, much progress has been made in designing ecological experiments along natural CO2/pH gradients systems, however great caution is required to assess ecological responses in submarine seeps, especially when their geochemistry is unknown.
Future directions
The study of the chemistry and ecology of volcanic CO2 seeps is an active field of research. Few shallow water submarine fields have been studied so far, and there is increasing need for a robust synthesis of this information, so future work should prioritize characterizing known (but unstudied) seeps, search for new ones, and assess how marine life acclimates or adapt to the high CO2 levels found at these sites. From a geochemical viewpoint, CO2 output estimates are available for only a few systems, and there is an opportunity to use newly developed technologies for in-situ, real-time mapping of pCO2 in seawater at high spatial and temporal resolution. From a biological point of view, progress is being made in our understanding of how coastal marine communities respond to rising CO2 levels. Over the next-decade CO2 seep research efforts should characterize trace metal interactions with biological processes and make use oflonger-term ecological experiments. Increased interaction and harmonization between geological, chemical and biological expertise looks set to provide improved insights into the responses of marine organisms to global OA.
Data availability
All data used in the manuscript are contained in the associated Supplementary Materials.
References
Agostini A, Harvey BP, Wada S, Kon K, Milazzo M, Inaba K, Hall-Spencer JM (2018) Ocean acidification drives community shifts towards simplified non-calcified habitats in a subtropical−temperate transition zone. Sci Rep 8:11354. https://doi.org/10.1038/s41598-018-29251-7
Albright R, Takeshita Y, Koweek D, Ninokawa A, Wolfe K, Rivlin T, Nebuchina Y, Young J, Caldeira K (2018) Carbon dioxide addition to coral reef waters suppresses net community calcification. Nature 555:516–519. https://doi.org/10.1038/nature25968
Alessi C, Giomi F, Furnari F, Sarà G, Chemello R, Milazzo M (2019) Ocean acidification and elevated temperature negatively affect recruitment, oxygen consumption and calcification of the reef-building Dendropoma cristatum early life stages: evidence from a manipulative field study. Sci Total Environ 693:133476. https://doi.org/10.1016/j.scitotenv.2019.07.282
Alt JC (1995) Seafloor processes inmid-ocean ridge hydrothermal systems. In: Humphris SE, Zierenberg RA, Mullineaux LS, Thomson RE (eds) Seafloor hydrothermalsystems: physical, chemical, biological, and geological interactions. American Geophysical Union, Washington, D.C., pp 85–114
Apostolaki ET, Vizzini S, Hendriks IE, Olsen YS (2014) Seagrass ecosystem response to long-term high CO2 in a Mediterranean volcanic vent. Mar Environ Res 99:9–15
Baross JA, Hoffman SE (1985) Submarine hydrothermal vents and associated gradient environments as sites for the origin and evolution of life. Origins Life 15:327–345
Becke R, Merkel B, Pohl T (2009) Mineralogical and geological characteristics of theshallow-water massive sulfide precipitates of Panarea, Aeolian Islands, Italy. In: Merkel B, Schipek M (eds) Research in shallow marine and fresh water systems. Freiberg online geology. TU Bergakademie, Freiberg, pp 94–100
Boatta F, D’Alessandro W, Gagliano AL, Liotta M, Milazzo M, Rodolfo-Metalpa R, Hall-Spencer JM, Parello F (2013) Geochemical survey of Levante Bay, Vulcano Island (Italy), a natural laboratory for the study of ocean acidification. Mar Pollut Bull 73:485–494
Brinkman TJ (2014) Suitability of volcanic vents at White Island, New Zealand for climate change research: effects on sea urchins and coralline algae (Doctoral dissertation, University of Otago)
Brodie J, Williamson CJ, Smale DA, Kamenos NA, Mieszkowska N, Santos R et al (2014) The future of the northeast Atlantic benthic flora in a high CO2 world. Ecol Evol 4:2787–2798. https://doi.org/10.1002/ece3.1105
Brown NEM, Milazzo M, Rastrick SPS, Hall-Spencer JM, Therriault TW, Harley CGD (2018) Natural acidification changes the timing and rate of succession, alters community structure, and increases homogeneity in marine biofouling communities. Glob Change Biol 24(1):112–127. https://doi.org/10.1111/gcb.13856
Butterfield A, Massoth GJ, McDuff RE, Lupton JE, Lilley MD (1990) Geochemistry of hydrothermal fluids from Axial Seamount hydrothermal emissions study vent field, Juan de Fuca Ridge: subseafloor boiling and subsequent fluid–rock interaction. J Geophys Res 95:12895–12921
Caliro S, Caracausi A, Chiodini G, Ditta M, Italiano F, Longo M, Minopoli C, Nuccio PM, Rizzo A (2004) Evidence of a new magmatic input to the quiescent volcanic edifice of Panarea, Aeolian Islands, Italy. Geophys Res Lett 31:1–5
Calosi P, Rastrick SPS, Graziano M, Thomas SC, Baggini C, Carter HA, Hall-Spencer J, Milazzo M, Spicer JI (2013) Acid-base and ion-regulation capacity-dependent distribution of sea urchins living near shallow water CO2 vents. Mar Pollut Bull 73:470–484. https://doi.org/10.1016/j.marpolbul.2012.11.040
Camp EF, Schoepf V, Mumby PJ, Hardtke LA, Rodolfo-Metalpa R, Smith DJ, Suggett DJ (2018) The future of coral reefs subject to rapid climate change: lessons from natural extreme environments. Front Mar Sci 5:4. https://doi.org/10.3389/fmars.2018.00004
Capaccioni B, Tassi F, Vaselli O (2001) Organic and inorganic geochemistry of low temperature gas discharges at the Baia di Levante beach, Vulcano Island, Italy. J Volcanol Geotherm Res 108:173–185
Capasso G, Inguaggiato S (1998) A simple method for the determination of dissolved gases in natural waters. An application to thermal waters from Vulcano Island. Appl Geochem 13(5):631–642
Caracausi A, Ditta M, Italiano F, Longo M, Nuccio PM, Paonita A (2005) Massive submarine gas output during the volcanic unrest off Panarea Island (Aeolian arc, Italy): inferences for explosive conditions. Geochem J 39:459–467
Cattano C, Claudet J, Domenici P, Milazzo M (2018) Living in a high CO2 world: a global meta-analysis shows multiple trait-mediated fish responses to ocean acidification. Ecol Monogr 88(3):320–335. https://doi.org/10.1002/ecm.1297
Cattano C, Agostini S, Harvey BP, Wada S, Quattrocchi F, Turco G, Inaba K, Hall-Spencer JM, Milazzo M (2020) Changes in fish communities due to benthic habitat shifts under ocean acidification conditions. Sci Total Environ 725:138501. https://doi.org/10.1016/j.scitotenv.2020.138501
Chen XG, Zhang HY, Xiaohu L, Chen C-TA (2016) The chemical and isotopic compositions of gas discharge from shallow-water hydrothermal vents at Kueishantao, offshore northeast Taiwan. Geochem J 50:341–355
Chiodini G, Marini L (1998) Hydrothermal gas equilibria: the H2O-H2-CO2-CO-CH4 system. Geochim Cosmochim Acta 62:2673–2687
Chiodini G, Cioni R, Marini L (1993) Reactions governing the chemistry of crater fumaroles from Vulcano Island Italy, and implications for volcanic surveillance. Appl Geochem 8:357–371
Chiodini G, Cioni R, Marini L, Panichi C (1995) Origin of fumarolic fluids of Vulcano Island Italy and implications for volcanic surveillance. Bull Volcanol 57:99–110
Connell SD, Doubleday ZA, Hamlyn SB, Foster NR, Harley CDG, Kelaher BP, Nagelkerken I, Sara G, Russell BD (2017) How ocean acidification can benefit calcifiers. Curr Biol 27:R95–R96
Connell SD, Doubleday ZA, Foster NR, Hamlyn SB, Harley CDG, Helmuth B, Kelaher BP, Nagelkerken I, Rodgers KL, Sarà G, Russell BD (2018) The duality of ocean acidification as a resource and a stressor. Ecology 88:1005–1010
Cornwall CE, Revill AT, Hall-Spencer JM, Milazzo M, Raven JA, Hurd CL (2017) Inorganic carbon physiology underpins macroalgal responses to elevated CO2. Sci Rep 7:46297
Cornwall CE, Comeau S, DeCarlo TM, Larcombe E, Moore B, Giltrow K et al (2020) A coralline alga gains tolerance to ocean acidification over multiple generations of exposure. Nat Clim Change 10:149
Crook ED, Kroeker KJ, Potts DC, Rebolledo-Vieyra M, Hernandez-Terrones LM, Paytan A (2016) Recruitment and succession in a tropical benthic community in response to in-situ ocean acidification. PLoS ONE 11(1):e0146707
Dando PR (2010) Biological communities at marine shallow-water vent and seep sites. In: Kiel S (ed) The vent and seep biota topics in geobiology. Springer, New York
de Ronde CEJ, Stucker VK (2015) Seafloor hydrothermal venting at volcanic arcs and Backarcs, Encyclopaedia of volcanoes, 2nd edn. Elsevier, Hoboken, pp 823–849
Di Napoli R et al (2016) Hydrothermal fluid venting in the offshore sector of Campi Flegrei caldera: a geochemical, geophysical, and volcanological study. Geochem Geophys Geosyst. https://doi.org/10.1002/2016GC006494
Doney SC, Busch DS, Cooley SR, Kroeker KJ (2020) The impacts of ocean acidification on marine ecosystems and reliant human communities. Annu Rev Environ Resour 45:83–112
Donnarumma L et al (2019) Environmental and benthic community patterns of the shallow hydrothermal area of Secca delle Fumose (Baia, Naples, Italy). Front Mar Sci 6:685. https://doi.org/10.3389/fmars.2019.00685
Ekstrom JA et al (2015) Vulnerability and adaptation of US shellfisheries to ocean acidification. Nat Clim Change 5(3):207
Enochs IC, Manzello DP, Donham EM, Kolodziej G, Okano R, Johnston L, Young C, Iguel J, Edwards CB, Fox MD, Valentino L, Johnson S, Benavente D, Clark SJ, Carlton R, Burton T, Eynaud Y, Price NN (2015) Shift from coral to macroalgae dominance on a volcanically acidified reef. Nat Clim Change 5:1083–1088. https://doi.org/10.1038/nclimate2758
Fabricius KE, Langdon C, Uthicke S, Humphrey C, Noonan S, De’ath G, Okasaki R, Muehllehner N, Glas MS, Lough JM (2011) Losers and winners in coral reefs acclimatized to elevated carbon dioxide concentrations. Nat Clim Change 1:165–169. https://doi.org/10.1038/nclimate1122
Fabricius KE, De’ath G, Noonan S, Uthicke S (2014) Ecological effects of ocean acidification and habitat complexity on reef-associated macroinvertebrate communities. Proc R Soc B 281(1775):20132479. https://doi.org/10.1098/rspb.2013.2479
Fabricius KE, Kluibenschedl A, Harrington L, Noonan S, De’ath G (2015) In situ changes of tropical crustose coralline algae along carbon dioxide gradients. Sci Rep 5:9537. https://doi.org/10.1038/srep09537
Fietzek P, Fiedler B, Steinhoff T, Körtzinger A (2014) In situ quality assessment of a novel underwater pCO2 sensor based on membrane equilibration and NDIR Spectrometry. J Atmos Oceanic Technol 31:181–196. https://doi.org/10.1175/JTECH-D-13-00083.1
Fischer TP, Chiodini G (2015) Volcanic, magmatic and hydrothermal gas discharges. Encyclopaedia of volcanoes, 2nd edn. Elsevier, Hoboken, pp 779–797
Foo SA, Byrne M, Ricevuto E, Gambi MC (2018) Oceanography and marine biology: an annual review. Oceanogr Mar Biol 56:237–310
Foster GL, Royer DL, Lunt DJ (2017) Future climate forcing potentially withoutprecedent in the last 420 million years. Nat Commun 8:14845. https://doi.org/10.1038/ncomms14845
Foustoukos DI, Seyfried WE (2007) Fluid phase separation processes in submarinehydrothermal systems. Rev Miner Geochem 65:213–239
Garilli V, Rodolfo-Metalpa R, Scuderi D, Brusca L, Parrinello D, Rastrick SPS, Foggo A, Twitchett RJ, Hall-Spencer JM, Milazzo M (2015) Physiological advantages of dwarfing in surviving extinctions in high-CO2 oceans. Nat Clim Change 5:678–682. https://doi.org/10.1038/nclimate2616
Garrard SL, Gambi MC, Scipione MB, Patti FP, Lorenti M, Zupo V, Paterson DM, Buia MC (2014) Indirect effects may buffer negative responses of seagrass invertebrate communities to ocean acidification. J Exp Mar Biol Ecol 461:31–38
Gaylord B, Kroeker KJ, Sunday JM, Anderson KM, Barry JP, Brown NE, Connell SD, Dupont S, Fabricius KE, Hall-Spencer JM, Klinger T, Milazzo M, Munday PL, Russell BD, Sanford E, Schreiber SJ, Thiyagarajan V, Vaughan MLH, Widdicombe S, Harley CDG (2015) Ocean acidification through the lens of ecological theory. Ecology 96:3–15. https://doi.org/10.1890/14-0802.1
German CR, Von Damm KL (2003) Hydrothermal processes. In: Turekian KK, Holland HD (eds) Treatise on geochemistry. Elsevier, Boboken, pp 145–180
Global CCS institute (2018) CCS Facilities Database. https://www.globalccsinstitute.com/resources/ccs-database-public/. Accessed 2 Apr 2019
Giggenbach WF (1980) Geothermal gas equilibria. Geochim Cosmochim Acta 44:2021–2032
Giggenbach WF (1987) Redox processes governing the chemistry of fumarolic gas discharges from White Island, New Zealand. Appl Geochem 2:143–161
Giggenbach WF (1988) Geothermal solute equilibria. Derivation of Na–K–Mg–Ca geoindicators. Geochim et Cosmochim Acta 52(12):2749–2765
Giggenbach WF (1997) The origin and evolution of fluids in magmatic hydrothermal systems. In: Barnes HL (ed) Geochemistry of hydrothermal ore deposits, 3rd edn. Wiley, New York, pp 699–736
Goffredo S, Prada F, Caroselli E, Capaccioni B, Zaccanti F, Pasquini L, Fantazzini P, Fermani S, Reggi M, Levy O, Fabricius K, Dubinsky Z, Falini G (2014) Biomineralization control related to population density under ocean acidification. Nat Clim Change 4:593–597
Gonzáles-Delgado S, Hernández JC (2018) The importance of natural acidified systems in the study of ocean acidification: what have we learned? Adv Mar Biol. https://doi.org/10.1016/bs.amb.2018.08.001
Hall-Spencer JM, Rodolfo-Metalpa R, Martin S, Ransome E, Fine M, Turner SM, Rowley SJ, Tedesco D, Buia MC (2008) Volcanic carbon dioxide vents show ecosystem effects of ocean acidification. Nature 454:96–99. https://doi.org/10.1038/nature07051
Hall-Spencer JM, Allen R (2015) The impact of CO2 emissions on ‘nuisance’ marine species. Res Rep Biodivers Stud 4:33–46
Hall-Spencer JM, Harvey BP (2019) Ocean acidification impacts on coastal ecosystem services due to habitat degradation. Emerg Top Life Sci 3(2):197–206
Hannington MD, de Ronde CEJ, Petersen S (2005) Modern sea-floor tectonics and submarine hydrothermal systems. Econ Geol 100:111–141
Hannington M, Jamieson J, Monecke T, Petersen S, Beaulieu S (2011) The abundance of seafloor massive sulfide deposits. Geology 39–12:1155–1158. https://doi.org/10.1130/G32468.1
Harvey BP, Gwynn-Jones D, Moore PJ (2013) Meta-analysis reveals complex marine biological responses to the interactive effects of ocean acidification and warming. Ecol Evol 3(4):1016–1030
Harvey BP, Agostini S, Kon K, Wada S, Hall-Spencer JM (2019) Diatoms dominate and alter marine food-webs when CO2 rises. Diversity 11(12):242
Harvey BP, McKeown NJ, Rastrick PS, Bertolini C, Foggo A, Graham H, Hall-Spencer JM, Milazzo M, Shaw PW, Small DP, Moore PJ (2016) Individual and population-level responses to ocean acidification. Sci Rep 4:20194. https://doi.org/10.1038/srep20194
Hedenquist JW, Lowenstern JB (1994) The role of magmas in the formation of hydrothermal ore deposits. Nature 370:519–527
Hedges LV, Gurevitch J, Curtis PS (1999) The meta-analysis of response rations in experimental ecology. Ecology 80:1150–1156
Hendriks IE, Duarte CM, Alvarez M (2010) Vulnerability of marine biodiversity to ocean acidification: a meta-analysis. Estuar Coast Shelf S 86(2):157–164
Hesselbo SP, Gröcke DR, Jenkyns HC, Bjerrum CJ, Farrimond P, Morgans Bell HS, Green OR (2000) Massive dissociation of gas hydrate during a Jurassic oceanic anoxic event. Nature 406:392–395. https://doi.org/10.1038/35019044
Kroeker K, Kordas RL, Crim RN, Singh GG (2010) Meta-analysis reveals negative yet variable effects of ocean acidification on marine organisms. Ecol Lett 13:1419–1434
Kroeker KJ, Micheli F, Gambi MC, Martz TR (2011) Divergent ecosystem responses within a benthic marine community to ocean acidification. Proc Natl Acad Sci USA 108:14515–14520. https://doi.org/10.1073/pnas.1107789108
Kroeker KJ, Kordas RL, Crim R, Hendriks IE, Ramajo L, Singh GS, Duarte CM, Gattuso JP (2013) Impacts of ocean acidification on marine organisms quantifying sensitivities and interaction with warming. Glob Change Biol 19:1884–1896. https://doi.org/10.1111/gcb.12179
IPCC (2019) Special report on the ocean and cryosphere in a changing climate. https://www.ipcc.ch/srocc/
Inguaggiato S, Mazot A, Diliberto IS, Inguaggiato C, Madonia P, Rouwet D, Vita F (2012) Total CO2 output from Vulcano island (Aeolian Islands, Italy). Geochem Geophys Geosyst 13(2):Q02012
Inoue S, Kayanne H, Yamamoto S, Kurihara H (2013) Spatial community shift from hard to soft corals in acidified water. Nat Clim Change 3:683–687. https://doi.org/10.1038/nclimate1855
Italiano F (2009) Hydrothermal fluids vented at shallow depths at the Aeolian islands: relationships with volcanic and geothermal systems. Freiberg Online Geol 22:55–60
Italiano F, Nuccio PM (1991) Geochemical investigation of submarine volcanic exalations to the east of Panarea, Aeolian Island, Italy. J Volcanol Geotherm Res 46:125–141
Italiano F, Nuccio PM, Sommaruga C (1984) Gas/steam and thermal energy release measured at the gaseous emissions of the Baia di Levante of Vulcano Island, Italy. Acta Vulcanol 5:89–94
Italiano F, Nuccio PM, Pecoraino G (1994) Fumarolic gas output at La Fossa di Vulcano crater. Acta Vulcanol 6:39–40
Jenkyns HC (2003) Evidence for rapid climate change in the Mesozoic-Palaeogene greenhouse world. Philos Trans R Soc Lond Ser A 361:1885–1916. https://doi.org/10.1098/rsta.2003.1240
Jenkyns HC (2010) Geochemistry of ocean anoxic events. Geochem Geophys Geosyst 11:3. https://doi.org/10.1029/2009GC002788
Johnson VR, Brownlee C, Rickaby REM, Graziano M, Milazzo M, Hall-Spencer JM (2013) Responses of marine benthic microalgae to elevated CO2. Mar Biol 160:1813–1824. https://doi.org/10.1007/s00227-011-1840-2
Lam VWY, Chavanich S, Djoundourian S, Dupont S, Gaill F, Holzer G, Isensee K, Katua S, Mars F, Metian M, Hall-Spencer JM (2019) Dealing with the effects of ocean acidification on coral reefs in the Indian Ocean and Asia. Region Stud Mar Sci 28:100560
Lemasson AJ, Hall-Spencer JM, Kuri V, Knights AM (2019) Changes in the biochemical and nutrient composition of seafood due to ocean acidification and warming. Mar Environ Res 143:82–92. https://doi.org/10.1016/j.marenvres.2018.11.006
Linares C, Vidal M, Canals M, Kersting DK, Amblas D, Aspillaga E, Cebrián E, Delgado-Huertas A, Díaz D, Garrabou J, Hereu B, Navarro L, Teixidó N, Ballesteros E (2015) Persistent natural acidification drives major distribution shifts in marine benthic ecosystems. Proc R Soc B 282:e20150587. https://doi.org/10.1098/rspb.2015.0587
Lu G-S, LaRowe DE, Fike DA, DruschelGK GWP III, Price RE et al (2020) Bioenergetic characterization of a shallow-sea hydrothermal vent system: Milos Island, Greece. PLoS ONE 15(6):e0234175. https://doi.org/10.1371/journal.pone.0234175
Lupton J, De Ronde C, Sprovieri M, Baker ET, Bruno PP, Italiano F, Walker S, Faure K, Leybourne M, Britten K, Greene R (2011) Active hydrothermal discharge on the submarine Aeolian Arc. J Geophys Res 116(2):B02102. https://doi.org/10.1029/2010JB007738
Martin S, Rodolfo-Metalpa R, Ransome E, Rowley S, Buia M-C, Gattuso J-P, Hall-Spencer JM (2008) Effects of naturally acidified seawater on seagrass calcareous epibionts. Biol Let 4:689–692
Martins M, Carreiro-Silva M, Martins GM, Barcelos e Ramos J, Viveiros F, Couto R, Parra H, Monteiro J, Gallo F, Silva C, Teodósio A, Guilini K, Hall-Spencer JM, Leitão F, Chícharo L, Range P (2020) Ervilia castanea (Mollusca, Bivalvia) populations adversely affected at CO2 seeps in the North Atlantic. Sci Total Environ 754:142044
Marty B, Alexander CMO, Raymond SN (2013) Primordial origins of earth’s carbon. Rev Miner Geochem 75:149–181. https://doi.org/10.2138/rmg.2013.75.6
Meyer KM, Kump LR (2008) Oceanic euxinia in Earth history: causes and consequences. Ann Rev Earth Planet Sci 36:251–288
Micheli F, Halpern BS (2005) Low functional redundancy in coastal marine assemblages. Ecol Lett 8(4):391–400. https://doi.org/10.1111/j.1461-0248.2005.00731.x
Milazzo M, Rodolfo-Metalpa R, San Chan VB, Fine M, Alessi C, Thiyagarajan V, Hall-Spencer JM, Chemello R (2014) Ocean acidification impairs vermetid reef recruitment. Sci Rep 4:4189. https://doi.org/10.1038/srep04189
Milazzo M, Alessi C, Quattrocchi F, Chemello R, D’Agostaro R, Gil J, Vaccaro AM, Mirto S, Gristina M, Badalamenti F (2019) Biogenic habitat shifts under long-term ocean acidification show nonlinear community responses and unbalanced functions of associated invertebrates. Sci Total Environ 667:41–48. https://doi.org/10.1016/j.scitotenv.2019.02.39
Mishra AK, Santos R, Hall-Spencer JM (2020) Elevated trace elements in sediments and seagrasses at CO2 seeps. Mar Environ Res. https://doi.org/10.1016/j.marenvres.2019.104810
Nagelkerken I, Russell BD, Gillanders BM, Connell SD (2015) Ocean acidification alters fish populations indirectly through habitat modification. Nat Clim Change 6:89–93
Paonita A, Federico C, Bonfanti P, Capasso G, Inguaggiato S, Italiano F, Madonia P, Pecoraino G, Sortino F (2013) The episodic and abrupt geochemical changes at La Fossa fumaroles (Vulcano Island, Italy) and related constraints on the dynamics, structure, and compositions of the magmatic system. Geochim Cosmochim Acta 120:158–178. https://doi.org/10.1016/j.gca.2013.06.015
Pichler T, Veizer J (1999) Precipitation of Fe(III) oxyhydroxide deposits from shallowwaterhydrothermal fluids in Tutum Bay, Ambitle Island, Papua New Guinea. Chem Geol 162:15–31. https://doi.org/10.1016/S0009-2541(99)00068-6
Pichler T, Veizer J, Hall GEM (1999a) The chemical composition of shallow-water hydrothermal fluids in Tutum Bay, Ambitle Island, Papua New Guinea and their effect on ambient seawater. Mar Chem 64(3):229–252
Pichler T, Veizer J, Hall GEM (1999b) The natural input of extremely high arsenic concentrations into a coral reef ecosystem by hydrothermal fluids and its removal byFe(III) oxyhydroxides. Environ Sci Technol 33:1373–1378
Pichler T, Biscéré T, Kinch J, Zampighi M, Houlbréque F, Rodolfo-Metalpa R (2019) Suitability of the shallow water hydrothermal system at Ambitle Island (Papua New Guinea) to study the effect of high pCO2 on coral reefs. Mar Pollut Bull 138:148–158
Price RE, Giovannelli D (2017) A review of the geochemistry and microbiology of marine shallow-water hydrothermal vents. Ref Module Earth Syst Environ Sci. https://doi.org/10.1016/B978-0-12-409548-9.09523-3
Price RE, Pichler T (2005) Distribution, speciation and bioavailability of arsenic in ashallow-water submarine hydrothermal system, Tutum Bay, Ambitle Island, PNG. Chem Geol 224:122–135. https://doi.org/10.1016/j.chemgeo.2005.07.017
Price RE, Savov I, Planer-Friedrich B, Buhring SI, Amend JP, Pichler T (2013) Processes influencing extremeAs enrichment in shallow-sea hydrothermal fluids of Milos Island, Greece. Chem Geol 348:15–26. https://doi.org/10.1016/j.chemgeo.2012.06.007
Price RE, LaRowe DE, Italiano F, Savov I, Pichler T, Amend JP (2015) Subsurface hydrothermal processes and the bioenergetics of chemolithoautotrophy at the shallow-sea vents off Panarea Island (Italy). Chem Geol 407:21–45. https://doi.org/10.1016/j.chemgeo.2015.04.011
Porzio L, Buia MC, Hall-Spencer JM (2011) Effects of ocean acidification on macroalgal communities. J Exp Mar Biol Ecol 400:278–287
Rainbow P (2002) Trace metal concentrations in aquatic invertebrates: why and sowhat? Environ Pollut 120:497–507. https://doi.org/10.1016/S0269-7491(02)00238-5
Rastrick SSP, Graham H, Azetsu-Scott K, Calosi P, Chierici M, Fransson A, Hop H, Hall-Spencer JM, Milazzo M, Thor P, Kutti T (2018) Using natural analogues to investigate the effects of climate change and ocean acidification on Northern ecosystems. ICES J Mar Sci 75:2299–2311. https://doi.org/10.1093/icesjms/fsy128
Riebesell U, Gattuso J-P (2015) Lessons learned from ocean acidification research. Nat Clim Change 5:12–14. https://doi.org/10.1038/nclimate2456
Royer DL, Berner RA, Park J (2007) Climate sensitivity constrained byCO2 concentrations over the past 420 million years. Nature 446:530–532
Russell BD, Connell SD, Uthicke S, Muehllehner N, Fabricius KE, Hall-Spencer JM (2013) Future seagrass beds: can increased productivity lead to increased carbon storage? Mar Pollut Bull 73(2):463–469. https://doi.org/10.1016/j.marpolbul.2013.01.031
Sedwick P, Stuben D (1996) Chemistry of shallow submarine warm springs in an arc-volcanic setting: Vulcano Island, Aeolian Archipelago, Italy. Mar Chem 53:147–161
Shamberger KEF, Cohen AL, Golbuu Y, McCorkle DC, Lentz SJ, Barkley HC (2014) Diverse coral com-munities in naturally acidified waters of a Western Pacific Reef. Geophys Res Lett 41:499–504. https://doi.org/10.1002/2013GL058489
Sommaruga C (1984) Le ricerche geotermiche svolte a Vulcano negli anni ’50. Rend Soc Ital Miner Petrol 39:355–366
Stumm W, Morgan JJ (1995) Aquatic chemistry: chemical equilibria and rates in natural waters, 3rd edn. Wiley, Hoboken
Suggett DJ, Hall-Spencer JM, Rodolfo-Metalpa R, Boatman TG, Payton R, Tye Pettay D, Johnson VR, Warner ME, Lawson T (2012) Sea anemones may thrive in a high CO2 world. Glob Change Biol 18:3015–3025
Sunday JM, Fabricius KE, Kroeker KJ, Anderson KM, Brown NE, Barry JP, Connell SD, Dupont S, Gaylord B, Hall-Spencer JM, Klinger T, Milazzo M, Munday PL, Russell BD, Sanford E, Thiyagarajan V, Vaughan MLH, Widdicombe S, Harley CDG (2017) Ocean acidification can mediate biodiversity shifts by changing biogenic habitat. Nat Clim Change 7:81–85. https://doi.org/10.1038/nclimate3161
Tarasov VG, Gebruk AV, Mironov AN, Moskalev LI (2005) Deep-sea and shallow-water hydrothermal vent communities: two different phenomena? Chem Geol 224:5–39
Tedesco D (1995) Fluid geochemistry at Vulcano Island: a change in the volcanic regime or continuous fluctuations in the mixing of different systems? J Geophys Res 100(B3):4157–4167
Teixidó N, Gambi MC, Parravacini V, Kroeker K, Micheli F, Ballesteros E (2018) Functional biodiversity loss along natural CO2 gradients. Nat Commun 9:5149
Thomsen J, Gutowska MA, Saphörster J, Heinemann A, Trübenbach K, Fietzke J, Hiebenthal C, Eisenhauer A, Körtzinger A, Wahl M, Melzner F (2010) Calcifying invertebrates succeed in a naturally CO2-rich coastal habitat but are threatened by high levels of future acidification. Biogeosciences 7:3879–3891. https://doi.org/10.5194/bg-7-3879-2010
Tittensor DP, Baco-Taylor AR, Brewin P, Clark MR, Consalvey M, Hall-Spencer JM, Rowden AA, Schlacher T, Stocks K, Rogers AD (2009) Predicting global habitat suitability for stony corals on seamounts. J Biogeogr 36:1111–1128
Tivey MK (2007) Generation of seafloor hydrothermal vent fluids and associated mineral deposits. Oceanography 20(1):50–65
Tunnicliffe V (1991) The biology of hydrothermal vents: ecology and evolution. Oceanogr Mar Biol Annu Rev 29:3119–3207
Turner LM, Ricevuto E, Massa-Gallucci A, Gambi MC, Calosi P (2015) Energy metabolism and cellular homeostasis trade-offs provide the basis for a new type of sensitivity to ocean acidification in a marine polychaete at a high-CO2 vent: adenylate and phosphagen energy pools versus carbonic anhydrase. J Exp Biol 218:2148–2151
Valsami-Jones E, Baltatzis E, Bailey EH, Boyce AJ, Alexander JL, Magganas A et al (2005) The geochemistryof fluids from an active shallow submarine hydrothermal system: Milos island, Hellenic Volcanic Arc. J Volcanol Geotherm Res 148(1–2):130–151
Vizzini S, Di Leonardo R, Costa V, Tramati CDD, Luzzu F, Mazzola A (2013) Trace element bias in the use of CO2 vents as analogues for low pH environments: implications for contamination levels in acidified oceans. Estuar Coast Shelf Sci 134:19–30
Vizzini S, Martínez-Crego B, Andolina C, Massa-Gallucci A, Connell SD, Gambi MC (2017) Ocean acidification as a driver of community simplification via the collapse of higher-order and rise of lower-order consumers. Sci Rep 7:4018. https://doi.org/10.1038/s41598-017-03802-w
Von Damm KL (1990) Seafloor hydrothermal activity: black smoker chemistryand chimneys. Annu Rev Earth Planet Sci 18:173–204. https://doi.org/10.1146/annurev.ea.18.050190.001133
Von Damm KL, Lilley MD, Shanks WC, Brockington M, Bray AM, O’Grady KM, Olson E, Graham A, Proskurowski G (2003) Extraordinary phase separation andsegregation in vent fluids from the southern East Pacific Rise. Earth Planet Sci Lett 206:365–378
Wignall PB, Twitchett RJ (1996) Oceanic anoxia and the end-Permian mass exitinction. Science 272:1155–1158
Wignall PB, Twitchett RJ (2002) Extent, duration, and nature of the Permian-Triassic superanoxic event. Geol Soc Am Spl Pap 356:395–413
Wittmann AC, Pörtner HO (2013) Sensitivities of extant animal taxa to ocean acidification. Nat Clim Change 3(11):995–1001
Zitoun R et al (2020) A unique temperate rocky coastal hydrothermal vent system (Whakaari–White Island, Bay of Plenty, New Zealand): constraints for ocean acidification studies. Mar Freshw Res 71(3):321. https://doi.org/10.1071/MF19167
Ziveri P, Passaro M, Incarbona A, Milazzo M, Rodolfo-Metalpa R, Hall-Spencer JM (2014) Decline in coccolithophore diversity and impact on coccolith morphogenesis along a natural CO2 gradient. Biol Bull 226(3):282–290. https://doi.org/10.1086/BBLv226n3p282
Funding
Open Access funding provided by Università degli Studi di Palermo within the CRUI-CARE Agreement. A.A. acknowledges funding support from the Alfred P. Sloan Foundation (Deep Carbon Observatory/DECADE project; UniPa-CiW subcontract 10881-1262) and the Italian Ministero Istruzione Università e Ricerca (MIUR, under Grant PRIN2017-2017LMNLAW).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Responsible Editor: Kelman Wieder
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aiuppa, A., Hall-Spencer, J.M., Milazzo, M. et al. Volcanic CO2 seep geochemistry and use in understanding ocean acidification. Biogeochemistry 152, 93–115 (2021). https://doi.org/10.1007/s10533-020-00737-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-020-00737-9