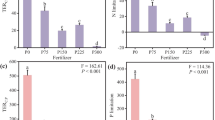
Abstract
Elevated CO2 (eCO2) can stimulate plant productivity and increase carbon (C) input to soils, but nutrient limitation restricts productivity. Despite phosphorus (P)-limited ecosystems increasing globally, it is unknown how nutrient cycling, particularly soil microbial extra cellular enzyme activity (EEA), will respond to eCO2 in such ecosystems. Long-term nutrient manipulation plots from adjacent P-limited acidic and limestone grasslands were exposed to eCO2 (600 ppm) provided by a mini-Free Air CO2 Enrichment system. P-limitation was alleviated (35 kg-P ha−1 y−1 (P35)), exacerbated (35 kg-N ha−1 y−1 (N35), 140 kg-N ha−1 y−1 (N140)), or maintained (control (P0N0)) for > 20 years. We measured EEAs of C-, N- and P-cycling enzymes (1,4-β-glucosidase, cellobiohydrolase, N-acetyl β-D-glucosaminidase, leucine aminopeptidase, and acid phosphatase) and compared C:N:P cycling enzyme ratios using a vector analysis. Potential acid phosphatase activity doubled under N additions relative to P0N0 and P35 treatments. Vector analysis revealed reduced C-cycling investment and increased P-cycling investment under eCO2. Vector angle significantly increased with P-limitation (P35 < P0N0 < N35 < N140) indicating relatively greater investment in P-cycling enzymes. The limestone grassland was more C limited than the acidic grassland, characterised by increased vector length, C:N and C:P enzyme ratios. The absence of interactions between grassland type and eCO2 or nutrient treatment for all enzyme indicators signaled consistent responses to changing P-limitation and eCO2 in both grasslands. Our findings suggest that eCO2 reduces C limitation, allowing increased investment in P- and N-cycle enzymes with implications for rates of nutrient cycling, potentially alleviating nutrient limitation of ecosystem productivity under eCO2.
Graphic abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Terrestrial ecosystems sequester nearly one-third of anthropogenic CO2 emissions (IPCC, 2013), with this uptake largely caused by elevated atmospheric CO2 concentrations (eCO2) increasing photosynthesis and carbon (C) storage in plant biomass and soils (IPCC, 2013). As atmospheric CO2 concentrations continue to rise, it is predicted that terrestrial ecosystems will sequester increasing amounts of C, constituting perhaps the single most important biogeochemical feedback to climate change (IPCC, 2013). Nutrient limitation of plant productivity, however, can severely restrict C uptake by ecosystems, and recent earth system model simulations (Zaehle et al. 2015) suggest that soil nitrogen (N) limitation could reduce potential C sequestration by up to 58%. However, phosphorus (P) limitation is also widespread (Elser et al. 2007; Fay et al. 2015) and may even surpass N limitation in its global extent (Du et al. 2020). Furthermore, ongoing N deposition is turning some N-limited ecosystems into P-limited ecosystems or increasing levels of P limitation in ecosystems already P-limited (Goll et al. 2012). Despite the spatial extent and increasing importance of P limitation, we know very little about how P limitation affects ecosystem responses to eCO2, limiting our ability to predict future rates of C uptake by the terrestrial biosphere (Zhang et al. 2014). In this context, grasslands are particularly important, representing 20% of global terrestrial net primary productivity (NPP) (Chapin et al. 2011) and being the most spatially extensive P-limited ecosystems in temperate regions (Jackson et al. 2002; Watson et al. 2011).
One indicator that soil microbes are experiencing changes in nutrient availability is their relative investment in extra cellular enzymes involved in cycling different nutrients (Luo et al. 2017, 2019). These enzymes break down complex soil organic matter (SOM) into organic monomers and minerals for microbial uptake (Allison and Vitousek 2005). Extra cellular enzyme activity (EEA) is linked to resource availability (Peng and Wang 2016) and is a good indicator of relative N versus P limitation (Sinsabaugh and Shah 2012): for example, P amendment of a P-limited has been shown to reduced acid phosphatase (AP) activity in rainforest soils (Turner and Wright 2014) and hay meadows (Colvan et al. 2001), whereas N addition to loess plateau grasslands has been shown to increase investment in P acquiring enzymes (Cui et al. 2020). These findings are consistent with evolutionary-economic theory which suggests that due to the cost of enzyme production, it is beneficial for an organism to reduce synthesis of an enzyme as the nutrient it is used to acquire becomes more available (Allison et al. 2010). However, since neither the acquisition of P nor N is independent of C (energy) supply, it should not be considered evidence of single resource ‘limitation’ (Sinsabaugh and Shah 2012). Rather, soil microbial respiration and growth generally increases with C addition (Rosinger et al. 2019) and so the relative activities of C, N and P enzymes should be considered as indicators of microbial limitation relative to the other nutrients and can be interpreted as colimitation of either N or P with C (Cui et al. 2019a, 2019b) and reference to microbial N and P limitation in this manuscript is done so with an implicit understanding that the is the case. Until very recently, few studies have investigated the effect of P limitation on soil enzyme activity (Marklein and Houlton 2012), but emerging work has shown microbial P and C limitation in grassland ecosystems with low rainfall (Cui et al. 2019a) and also in ‘degraded’ grasslands where C and P limitation persists for several decades after restoration measures have been put in place (Cui et al. 2019b; Deng et al. 2019; Yang et al. 2020).
To predict long-term changes in productivity, it is essential to understand how eCO2 influences C allocation to different nutrient acquisition strategies, and in the case of P-limited ecosystems, the extent to which P limitation can be alleviated (Hoosbeek 2016). Under eCO2, increased photosynthesis is expected to see more C entering the soil (Hungate et al. 1997), which may then become available for the synthesis of C-rich enzymes such as AP (Thaller et al. 1997). Previous research has suggested that potential enzyme activities can increase under eCO2 (Austin et al. 2009; Souza et al. 2017), including in grasslands (Ebersberger et al. 2003; Guenet et al. 2012). Importantly, in an N-fertilised grassland, it was potential AP activity that increased the most in response eCO2, suggesting that eCO2 may allow for increased investment by microbes into the acquisition of a potentially limiting nutrient (Guenet et al. 2012).
To further test whether eCO2 can increase microbial investment in P cycling, we made use of a unique experimental resource constituting two grassland ecosystems (a limestone and an acidic grassland) in which plant productivity has been shown to be P-limited (Carroll et al. 2003) and soil N and P availability has been manipulated for ~ 20 years (Phoenix et al. 2003). The long-term nutrient additions provide grasslands with four levels of P limitation of plant productivity: alleviated P limitation (through P addition), natural P limitation (no nutrient treatment) and two levels of exacerbated P limitation (through low and high rates of N addition) (Phoenix et al. 2004). Furthermore, the two grasslands differ in their relative availability of different soil P sources (mineral and organic), with lower organic P contents in the limestone grassland soil compared to the acidic (Horswill et al. 2008). Despite the understanding of plant nutrient limitation in these systems, hitherto no investigation of microbial response to nutrient addition has been conducted. Thus, we were able to investigate the potential for plant alleviation of P limitation under different biogeochemical conditions, with any up-regulation of microbial AP activity potentially allowing for greater access to the large organic P pools in the acidic grassland compared to the limestone grassland.
Mesocosms were extracted from the nutrient manipulation experiment and subjected to ambient or eCO2 (600 ppm) using mini-FACE rings, with the soils used in this study being sampled after the first full growing season of CO2 fumigation. We conducted assays on five enzymes key for C, N and P cycling: 1,4- β -glucosidase (BG; C cycle enzyme), cellobiohydrolase (CB, C cycle), N-acetyl β-D-glucosaminidase (NAG, N cycle), leucine aminopeptidase (LAP, N cycle), and acid phosphatase (AP, P cycle). Assays were designed to measure potential EEA, which is a reflection of microbial investment in element cycling and not the rate of nutrient cycling itself. Therefore, high potential EEA is likely to indicate low availability and cycling of the relevant nutrient (i.e. a high microbial demand for that element and high investment in its acquisition). In addition to the specific activities of individual enzymes, EEA ratios of C:N:P acquiring enzymes (enzyme stoichiometry) can be interpreted to investigate the relative investment in C, N and P cycling by soil microbes (Sinsabaugh and Shah 2012). We then used these ratios in a vector analysis in a single, quantifiable comparison of relative nutrient investment strategies of soil microbes (Moorhead et al. 2013, 2016). We hypothesised:
-
1
Under eCO2, soil microbes will reduce investment in C cycling enzymes.
-
2
eCO2 would result in increased investment in P cycle enzymes.
-
3
Potential activity of AP would increase with decreasing P availability.
-
4
The response of potential EEA’s to P addition and eCO2 would be greater in the acidic grassland where there was relatively more organic P.
Methods and materials
Experimental design
Intact soil-turf monoliths were taken from a long-term grassland nutrient manipulation experiment that was established in 1995 at Wardlow, Peak District National Park, UK (Phoenix et al. 2003). The limestone grassland (NVC classification Festuca-Avenula CG2d) occurs on a shallow ranker (~ 10 cm A horizon down to limestone parent material) that is transitioning from a humic rendzina due to leaching. The acidic grassland (Festuca-Agrostis-Galium U4e) is on a cryptic podzol, with an organic-rich A horizon up to 15 cm deep above a mineral horizon extending to at least 70 cm depth (though rooting and biological activity is largely limited to the top 15 cm). On each grassland, replicate 9 m2 experimental plots have received either no treatment (natural P limitation, application of distilled water only (P0N0)), monthly applications of P at 35 kg P ha−1 y−1 [providing alleviation of P limitation; NaH2PO4.H2O solution applied (P35)], or N at 35 and 140 kg N ha−1 y−1 [providing two levels of exacerbated P limitation; NH4NO3 solution applied (N35 and N140, respectively)], amendments which were designed to manipulate the relative P and N availability to the ecosystem. The nutrient additions have strongly affected the absolute and relative availabilities of N and P in the soils. P addition increased extractable phosphate-P by ca. 50% in both grasslands (unpublished data), while N addition has been shown to have increased extractable N (nitrate plus ammonium) by ca. 370% and 350% in the acid and limestone grasslands, respectively (O'Sullivan et al. 2011). In the limestone grassland, N addition reduced extractable P availability by ~ 30%, but N addition increased extractable P by ~ 40% in the acidic grassland (unpublished data). Therefore, N addition resulted in an absolute and relative reduction in P availability in the limestone grassland, and a relative reduction in P availability in the acidic grassland (i.e. the relative increase in N availability was 10 times greater than the relative increase in P availability).
Ten replicate monoliths (0.35*0.35 m) were removed from each treatment at Wardlow during February and March 2017. Monoliths were excavated to 10 cm (the full soil profile to bedrock) in the limestone grassland and 20 cm in the acidic grassland (below the main rooting depth) and were transported to the Bradfield Environment Laboratory research station, also in the Peak District National Park, were they were transplanted into polypropylene mesocosm boxes. Since roots readily reach the underlying limestone in shallow rendzina soils, a base of limestone pieces was applied to the bottom of the limestone mesocosms. Limestone chippings were sourced to be the correct limestone type for the field location (Bee Low limestone, Dove Hole Quarry, Derbyshire UK).
At the Bradfield research station, mesocosms were housed outside, embedded in the native soil to ensure they were thermally buffered. The mesocosm sides and base were fully enclosed so there was no direct contact with the surrounding soils. Each mesocosm was free draining through a base of mesh voile (to stop particulate loss and root outgrowth, or ingrowth of roots from surrounding plants). The Bradfield research station (ca. 390 m asl) is less than 20 km from Wardlow (ca. 350 m asl) and has similar climate.
Mesocosms were assigned to either elevated CO2 (eCO2) or ambient CO2 treatments, with eCO2 provided by CO2 fumigation from a miniFACE system (Miglietta et al. 2001), such that each miniFACE ring or control had one each of the four nutrient treatments from each grassland (8 mesocosms in total). Briefly, the miniFACE system comprised five 1.6 m diameter FACE rings and five control rings, with each ring consisting of PVC tubes with laser drilled micro-holes. The processors in the FACE ring control units received CO2 information from sensors (GTM222, Vaisala, Finland) installed in the centre of each ring. Microprocessors, linked to automated pressure regulators, controlled the pressure inside the releasing pipes and therefore delivery of CO2 to the rings. Fumigation started in April 2018, with fossil fuel-derived CO2 supplied by BOC Cryospeed (BOC Limited, Manchester, UK). During the hours of daylight, CO2 enrichment was set to a target of 600 ppm, which continued until the end of October 2018, and ambient CO2 averaged 400 ppm across the study. For five weeks, during June and July, the target ppm was reduced to 550 ppm due to a CO2 supply shortage in the UK.
Soil sampling
In late September 2018, five replicate soil cores (diameter 2 cm) were sampled at random positions within each mesocosm and, in the acidic mesocosms, cores were separated into A and B horizons. The replicate cores were well mixed and first passed through a 10 mm sieve to remove large roots and stones. Approximately 4 g of sieved soil was oven dried at 105 °C to calculated gravimetric moisture content. Roots were picked from the remaining undried soil which was then passed through a 2 mm sieve and stored at 4 °C for no more than 5 weeks prior to laboratory analysis.
Laboratory analyses
From this fresh soil, fluorimetric assays were conducted to measure the potential activity of CB, BG, LAP, NAG and AP. Preliminary incubation of the soils at pH 4.0, 5.0 and 6.0 indicated that pH 5.0 gave the most replicable results as shown by the smallest variance (Table S1). Soil suspensions were prepared by adding 1 g fresh soil to 125 ml of 50 mM sodium acetate buffer (pH 5.0) and sonicating for 120 s (VXC 130 Ultrasonic Processor, Sonics and Materials Inc., Connecticut, USA, intensity 85%, equivalent to ca. 6000 kJ), and were then continuously stirred at room temperature prior to loading into a dark 96 well microplate (Greiner Bio-One Ltd., S655077, 96 × 600 µL, flat bottom). Duplicate standard wells of 4-Methyllumbelliferone (MUF) and 7-Amino-4-methylcoumarin (AMC) with concentrations ranging from 0 μM to 50 μM were included in each plate and soil suspensions were measured in triplicate. Fluorescence was measured at 30 °C using a microplate fluorometer (FLUOstar Optima Fluorescence meter with combination optics, BMG LABTECH, Offenburg Germany) with 360 nm excitation and 460 nm emission filters. Enzyme activity was calculated according to the equations in German et al. (2011):
where
The emission coefficient calculated using
The quench coefficient was calculated as
The activity was then corrected for the gravimetric water content (%) to express enzyme activity as nmol g−1 dry soil h−1.
Data analyses, enzyme activity ratios and vector analysis
The ratios of EEA of C (BG), N (NAG + LAP) and P (AP) cycling enzymes in the A horizons of both grasslands were calculated as proportional ratios [e.g. C:P = BG/(BG + AP)]. Proportional ratios are presented here due to the statistical limitations of simple ratios (Moorhead et al. 2016). As in previous studies (e.g. Moorhead et al. 2016), only BG activities were included in the C to nutrient cycle enzyme ratios, but C to nutrient cycle ratios based on combined BG and CB activities (not shown) yielded near identical relationships and statistical significances. The angle and the vector of the enzyme ratios were calculated as the square root of the sum of the squared values of x and y, where x represents untransformed C:P enzyme activities and y represents untransformed C:N activities (Moorhead et al. 2016).
Mixed effects models were used to analyse the effect of eCO2, nutrient treatment, grassland and horizon, with block as a random factor, on the activity of the five enzymes. No significant interaction between horizon and either eCO2 or nutrient treatment was found in the acidic grassland, therefore only the EEA activity from the A horizon was used for vector analysis. Where necessary, data were log transformed and three-way analysis of variance was then conducted to test for effects of eCO2, nutrient treatment and grassland type on enzyme ratios, vector length and vector angle. Differences between group means were tested using a post hoc Duncan's Multiple Range test. All analyses were performed using SAS 9.4 (SAS Institute, Cary, NC, USA).
Results
Enzyme activities
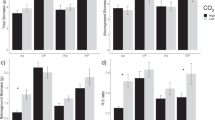
Differences in the activities of several enzymes were seen between nutrient additions, grassland type and horizon (Fig. 1, Table S2). A significant effect of nutrient treatment was seen in EEA of AP (F[2,12] = 5.10, p < 0.02), with the highest values seen in the N140 and N35 treatments, around 100% greater than in the P0N0 and P35 mesocosms (Fig. 1m–o). There was also a near-significant change (F[3,12] = 3.31, p < 0.058) in LAP activity, with activity increasing with P limitation by nearly 30% from 29 nmol g−1 h−1 in the P35 treatment to 21 nmol g−1 h−1 in the N140 treatment (Fig. 1j–l). The nutrient treatments did not have an effect on EEA of the other three enzymes. There was, however, a significant interaction between nutrient and grassland in EEA of both NAG (F[3,80] = 4.28, p < 0.01) and LAP (F[3,80] = 4.42, p < 0.01), where EEA of LAP declined with increasing P limitation (greatest under P35 addition, lowest under N140), and NAG remained fairly constant in the limestone grassland, but less clear, if not opposing effects for both enzymes was seen in the acidic grassland (Fig. 1g–i).
Enzyme activities of β-glucosidase (BG: a–c), cellobiohydrolase (CB, d–f), N-acetyl-β-D-glucosaminidase (NAG, g–i), leucine aminopeptidase (LAP, j–l) and acid phosphatase (AP, m–o) in acidic grassland horizons (left and middle columns) and limestone (right hand column) under ambient (black) and eCO2 (grey). Nutrient treatments on the horizontal axis are ordered in increasing P limitation from left to right. Lower case letters denote significant differences (p < 0.05) between nutrient treatments
The overall mean EEA of LAP was 41 nmol g−1 h−1 in the limestone grassland, more than 200% that in the acidic, 15 nmol g−1 h−1 (Fig. 1j–l; F[1,4] = 35.70, p < 0.004). Within the acidic grassland, EEA tended to be greater in the A horizon compared to the B horizon, with activity 35% greater for NAG (F[1,80] = 5.26, p < 0.03), 66% BG (F[1,4] = 11.77, p < 0.001), 30% LAP (F[1,80] = 4.2, p < 0.04) and 53% greater for AP (F[1,80] = 12.04, p < 0.001) respectively, although there was no interaction between horizon with either CO2 or nutrient treatment. Although an overall trend of higher EEA under ambient CO2 was seen in all enzymes except AP, no significant differences between CO2 treatments were detected.
Enzyme ratios
There was a strong nutrient effect ((F[3,119] = 26.27, p < 0.001) on C:P enzyme ratio, which was highest under P addition, declining by ca. 50% from 0.39 to 0.21 with increasing amounts of N addition (Fig. 2d–f). The ratio of N:P cycling enzymes also declined significantly by ca. 50% from 0.28 to 0.16 with additional N (F[3,119] = 14.35, p < 0.001, Fig. 2g–i). Nutrient addition did not alter the C:N enzyme ratio.
The mean (n = 10, ± 1SE) proportional ratios of C (BG), N (LAP + NAG) and P (AP) cycling enzymes from the limestone and acid grasslands, under ambient (A) and eCO2 (E), from four different nutrient treatments (P35 = 35 kg P ha-1 y−1, P0N0 = control, N35 = 35 kg N ha−1 y−1, N140 = 140 kg N ha−1 y−1). Significant differences between nutrient treatment group means are indicated by different letters (p < 0.05, ANOVA, post-hoc test Duncan's Multiple Range test) and an overall significant effect of eCO2 shown with * (p < 0.05). Between grasslands, significant differences (p < 0.001) were observed in C:N and C:P ratios but not for N:P ratios
The overall C:N cycling enzyme ratio increased by 15% in the limestone grassland compared to the acid grassland (F[1,119] = 14.52, p < 0.001; Fig. 2a–c), from 0.57 to 0.66. The C:P ratio was also higher in the limestone grassland than the acidic (F[1,119] = 19.05, p < 0.001; Fig. 2g–i), with a ca. 20% increase from 0.27 to 0.33, but N:P ratio did not differ between grasslands.
The proportional ratios of C to N cycling enzymes (F[1,119] = 4.71, p < 0.04; Fig. 2a–c), and C to P cycling enzymes (F[1,119] = 5.56, p < 0.03; Fig. 2d–f), decreased significantly under eCO2 from 0.62 to 0.58, and from 0.30 to 0.26, for C:N and C:P enzymes respectively. However, there was no effect of eCO2 on N:P enzyme ratio (Fig. 2g–i).
Vector analysis of microbial nutrient limitation
Vector angle decreased as P limitation increased (Fig. 3 panel b) and a decrease in vector length was seen under eCO2 (Fig. 3 panel a). These trends were consistent across the two grasslands (Fig. 4).
extra-cellular enzyme stoichiometry of relative activities of C:P acquisition enzymes and C:N enzymes in the limestone and acidic grasslands (A horizon only) combined showing: panel a 95% confidence intervals (dotted and dashes lines) of ambient and eCO2 treatments; and b 95% confidence interval (dotted and dashed lines) of four nutrient treatments (P0N0 = control, N35 = low nitrogen (35 kg ha−1 y−1), N140 = high nitrogen (140 kg ha−1 y−1) and P35 = phosphorus (35 kg ha−1 y−1). The solid black line represents the 1:1 line and is equivalent to a vector angle of 45°, with P limitation increasing with angle (distance above line) and N limitation with increasing distance below the line. C limitation increases with distance from the origin (vector length)
Comparison of vector length and angle between different nutrient treatments, levels of CO2 (black = ambient, grey = eCO2) and grassland (acidic, a and c, limestone b and d). Increasing vector angle indicates greater P limitation, decreasing N limitation. Vector length indicates relative C limitation (longer = more C limited). Bars with different letters within each are statistically different (p < 0.05, ANOVA, post-hoc test Duncan's Multiple Range test), ** denotes overall significant difference between levels of CO2 (p < 0.01). A significant difference between grasslands was seen in vector length (p < 0.001)
Vector angle- N and P limitation
Vector angle significantly differed between nutrient treatments (F[3,79] = 23.70, p < 0.001), increasing from 57.5° under P addition to 71.6° under N140 (Fig. 4a, b). There was no significant difference in vector angle between the two grasslands, but the effect of eCO2 approached significance (F[1,79] = 3.10, p < 0.09) with a trend of a higher vector angle with eCO2, suggesting increased investment in P cycling enzymes.
Vector length- C limitation
A significant nutrient effect was seen on vector length (F[3,79] = 6.68, p < 0.001), which increased sequentially from the N140 treatment, through N35 to P35 (Fig. 3 panel b, Fig. 4a, b), indicating greater relative investment in C cycle enzymes as P limitation was alleviated. Vector length was also 10% greater in the limestone compared to the acidic grassland (F[1,79] = 10.18, p < 0.01) (Fig. 4a, b), indicative of greater relative C limitation in the limestone grassland. Furthermore, vector length was nearly 10% shorter under eCO2, F[1.79] = 8.13, p < 0.01 (Fig. 3 panel a, Fig. 4a, b), suggesting a relative decrease in C cycle investment by the microbes.
[Table 1 here].
Discussion
This first assessment of the effects of elevated CO2 across different levels of P limitation has, through vector analysis, revealed significant reductions in soil C cycle enzyme investment and increases in P cycle enzyme investment under eCO2. It is important to acknowledge that these findings are, at this point, short term responses, but they suggest that under eCO2 microbes could increase relative investment in P cycle enzymes, facilitated by a reduced need to invest in C cycling enzymes (Table 1).
Effect of CO2 on microbial energy and nutrient limitation
Though our results showed no effect of eCO2 on the EEA of individual enzymes, effects became apparent when the ratios of EEA were calculated, with a significant reduction seen in C:N and C:P activity under eCO2. In previous studies, contrasting results have been observed in terms of the responses of individual enzymes to eCO2. These range from no change in EEA (Hasegawa et al., 2016), to increases of a single enzyme’s activity (Austin et al. 2009; Dorodnikov et al. 2009; Ebersberger et al. 2003; Guenet et al. 2012) or even all C, N and P cycling enzymes (Souza et al. 2017). Our ratio analysis, on the other hand, revealed consistent biological responses to eCO2 in both grasslands. These responses were further clarified by considering the activity of C, N and P enzymes together, in a vector analysis. Rosinger et al. (2019) suggest that enzyme stoichiometry reflects relative N or P limitation while co-limited by C supply, and we interpret our findings with that understanding in mind. Whilst such an understanding is commonplace in recent literature (Cui et al. 2019a, b, 2020), an alternative, conceptual model suggests that using the ratio of BG to NAG + LAP to infer relative C:N limitation may not be suitable as microbes may use chitin, a substrate of NAG, as an alternative C source (Mori 2020). In this scenario increasing NAG activity may in fact reflect increased C limitation. We explored this possibility by also using our data to calculate C:N ratio using BG to LAP alone (Figure S1). As this showed similar patterns and statistical significance to BG to NAG + LAP, we present and interpret C:N ratios using both NAG and LAP.
To our knowledge, ours is the first study to conduct enzyme stoichiometry vector analysis under eCO2. The reduction in vector length under eCO2 supported our hypothesis that microbes were investing relatively less in C cycling enzymes, which is likely due to there being more labile C available (Hungate et al. 1997). We would expect to see an increase in below-ground C unputs under eCO2, either as a result of an increased rate of root exudation (Philips et al. 2006), an increase in root biomass, or a combination of both (Cotrufo and Gorissen 1997). The effect of eCO2 on vector length in our study was less pronounced in the N140 treatment compared to the other amendments, perhaps reflecting a previous finding that N addition reduces labile C availability to microbes (Chen et al. 2018). The trend of increasing relative P investment (increased vector angle) under eCO2 suggests that microbes are able to use the additional C, and perhaps some of the N saved from reduced investment in C cycle enzymes, for synthesis of P-acquiring enzymes in an attempt to secure more of this low availability nutrient. The increased relative microbial investment in P acquisition, enabling increased mineralisation in these naturally P-limited ecosystems, therefore provides a mechanism by which eCO2 may alleviate plant and ecosystem P limitation.
Effect of long-term nutrient manipulation on P limitation
Whilst the potential activities of C and N cycling enzymes were lower than those seen in grasslands elsewhere, AP activity was similar (Herold et al. 2014). The increase in potential acid phosphatase activity (AP) in the soils under N addition in our study is consistent with previous work at the site which observed an increase in phosphomonoesterase (PME) activity on root surfaces in these grasslands following N addition (Johnson et al. 1998; Phoenix et al. 2004); this has also been seen in other P-limited grasslands (Jing et al. 2016). The increase in AP activity between the plots receiving N, to exacerbate P limitation and those not receiving N, partly supports the hypothesis that an increase in P limitation would stimulate AP activity. An analogous effect has been demonstrated in N-limited grasslands, where AP activity increased with N addition (Dong et al. 2019). Whilst in a very different ecosystem, our results are also broadly in agreement with the observed responses of EEA to nutrient addition in very low P-availability tropical soils. In such ecosystems, increasing P limitation has been shown to increase the relative investment in P acquiring enzymes (Waring et al. 2014), and long-term N addition has been shown to stimulate AP activity (Wang et al. 2018).
That AP activity did not differ between the control and P plots might appear to counter our third hypothesis, until enzyme stoichiometry is considered, which showed a clear differentiation between the relative P investment in the P and control plots. A better indicator of microbial response to nutrient availability, the CNP stoichiometry of EEA, through vector analyses illustrate increased investment in P cycling enzymes relative to N cycling enzymes with decreasing relative P availability, consistent with our second hypothesis. Strikingly, despite the long-term P addition, the vector angle was > 45°, the threshold above which it is commonly considered that N limitation shifts to P limitation (Cui et al. 2019a, b; Deng et al. 2019; Sinsabaugh et al. 2016). It may be more appropriate to consider vector angle as a continuum from N limitation (0°) to P limitation (90°) rather than use the 45° angle as a strict delineation (Loeppmann et al. 2016). Regardless, these findings indicate that even with P addition, P limitation may still be important in shaping microbes’ enzyme investment. This analysis also showed that with N addition there was decreasing investment in C cycle enzymes (reduced vector length) occurring alongside an increased relative investment in P-acquiring enzymes (increased vector angle); this may indicate that when the availability of a key nutrient increases, in this case P, microbes increase their demand for C, and vice versa. This is consistent with a recent meta-analysis (Chen et al. 2019) which demonstrated a similar effect of increasing C demand when adding N to low N-availability systems, consistent with the prediction of Kopacek et al. (2013). Alternatively, it may be evidence of changes in mycorrhizal activity: under P addition, which is known to supress mycorrhizal infection (Abbott et al. 1984), the symbiosis through which plant C is ‘traded’ for P can be reduced, hence C availability may decline (increased vector length) with P addition. Conversely, under lower P availability (N35 and N140 treatments), the increased microbial investment in P cycling enzymes (increased vector angle) may reflect increased incidence of mycorrhizal symbiosis, thus the transfer of plant C results in a lower microbial investment in C cycling enzymes (reduced vector length). A third possibility is that nutrient addition alters the stoichiometry of soil substrates. If this shift results in soil substrate stoichiometry which is closer the microbial critical ratio, microbes will be able to increase their carbon use efficiency (CUE) (Sinsabaugh et al. 2016). In such circumstances, microbes would require less C to acquire N and P and may therefore reduce investment in C cycling enzymes.
Whatever the explanation, if microbes need to invest less in C-cycle enzymes this may allow them to invest more in cycling the P, the element that most strongly limits plant productivity in these grasslands. This suggests that, in time, under eCO2 microbes may alleviate P limitation of plants and help to sustain increased NPP (Capek et al. 2018). Based on the differing relative demand of plants and microbes for N and P (microbial biomass is very rich in P), plants could be released from P limitation while microbes are still investing strongly in P-cycle enzymes (Capek et al. 2018). In fact, based on the stoichiometric nutrient limitation concept of Capek et al. (2018), the reduced investment in C-cycle enzymes by the microbes may be driven by plants, exchanging exudates for microbially-acquired P (Meier et al. 2015).
Comparison of enzyme activity between grasslands
The EEA of LAP was higher in the limestone mesocosms, and there was a similar, non-significant trend in BG activity. More informative was the increased ratio of C:N and C:P enzyme activity in the limestone grassland, which contains less soil organic matter. This was also reflected in a greater vector length (relative C limitation) seen in the limestone grassland. Despite this, we found consistent responses of enzyme stoichiometry to eCO2 and nutrient additions across both grasslands. Thus, we have shown that, regardless of grassland type, eCO2 provides a mechanism through which microbes may be able to invest more in producing nutrient cycle enzymes. We might, therefore, expect to see a similar response to environmental change in P-limited grassland ecosystems across the globe.
Implications for climate change, nutrient cycling and soil C storage
Our findings also have implications for the importance of accounting for nutrient limitation in process-based models which aim to predict ecosystem NPP. Few models couple C, N and P cycling with regard to productivity, and those that do are yet to predict responses to eCO2 (Davies et al. 2016). Ecosystem models designed to predict C allocation from NPP under eCO2 generally neglect changes to rhizosphere exudation (De Kauwe et al. 2014), a key C flux that can influence microbial EEA, which we have shown may be an important climate change-induced feedback to plant nutrient limitation. As our study has focussed solely on EEA in soils, the key next step would be to establish in the longer term whether greater investment in P cycle enzymes promotes more rapid P cycling and thus alleviation of nutrient limitation to plants, or whether it becomes just a symptom of increasing P limitation within the ecosystem. Future work is needed to link EEA to changes in ecosystem pools of C, N and P (e.g. plant biomass, soil and soil microbial biomass) under eCO2 to ascertain which of these alternative explanations is most important. Such work should focus on quantifying fluxes between nutrient pools and the regulating processes, at the larger scale such as N cycling and the molecular scale such as through gene expression and microbial community analysis.
Conclusion
Through our manipulations, which both alleviate and exacerbate P limitation of plant productivity in two contrasting grasslands, we provide evidence that soil microbes increase their relative investment in extra cellular enzymes (their extracellular enzyme stoichiometry), according to the changes in P limitation. We have also shown that, even after a single growing season at eCO2, soil microbes alter their nutrient investment strategies through changes in their enzyme stoichiometry. This suggests that eCO2 will play an important role in altering the relative nutrient limitation of ecosystems. With an increase in atmospheric CO2, soil microbes reduced investment in C cycling enzymes in favour of P or N cycling enzymes in both grasslands and this response represents a potential microbial mechanism that could help alleviate nutrient limitation under future eCO2 scenarios. The extent to which this response can promote a long-term increase in soil nutrient availability requires further investigation.
Data availability
Data will be available from the Environmental Information Data Centre, https://eidc.ac.uk/.
Code availability
Not applicable.
References
Abbott LK, Robson AD, Deboer G (1984) The effect of phosphorus on the formation of hyphae in soil by the vesicular arbuscular mycorrhizal fungus Glomus fasciculatum. New Phytol 97(3):437–446
Allison SD, Vitousek PM (2005) Responses of extracellular enzymes to simple and complex nutrient inputs. Soil Biol Biochem 37(5):937–944
Allison SD, Weintraub MN, Gartner TB, Waldrop MP (2010) Evolutionary-economic principles as regulators of soil enzyme production and ecosystem function. In: Shukla G, Varma A (eds) Soil enzymology. Springer, New York, pp 229–243
Austin EE, Castro HF, Sides KE, Schadt CW, Classen AT (2009) Assessment of 10 years of CO2 fumigation on soil microbial communities and function in a sweetgum plantation. Soil Biol Biochem 41(3):514–520
Capek PT, Manzoni S, Kastovska E, Wild B, Diakova K, Barta J, Schnecker J, Blasi C, Martikainen PJ, Alves RJE, Guggenberger G, Gentsch N, Hugelius G, Palmtag J, Mikutta R, Shibistova O, Urich T, Schleper C, Richter A, Santruckova H (2018) A plant-microbe interaction framework explaining nutrient effects on primary production. Nat Ecol Evol 2(10):1588–1596
Carroll JA, Caporn SJM, Johnson D, Morecroft MD, Lee JA (2003) The interactions between plant growth, vegetation structure and soil processes in semi-natural acidic and calcareous grasslands receiving long-term inputs of simulated pollutant nitrogen deposition. Environ Pollut 121(3):363–376
Chapin FS, Matson PA, Vitousek PM (2011) Nutrient cycling. In: Chapin FS, Matson PA, Vitousek PM (eds) Principles of Terrestrial Ecosystem Ecology. Springer, New York, pp 259–296
Chen H, Li DJ, Mao QG, Xiao KC, Wang KL (2019) Resource limitation of soil microbes in karst ecosystems. Sci Total Environ 650:241–248
Chen H, Li DJ, Zhao J, Zhang W, Xiao KC, Wang KL (2018) Nitrogen addition aggravates microbial carbon limitation: Evidence from ecoenzymatic stoichiometry. Geoderma 329:61–64
Colvan SR, Syers JK, O’Donnell AG (2001) Effect of long-term fertiliser use on acid and alkaline phosphomonoesterase and phosphodiesterase activities in managed grassland. Biol Fertil Soils 34(4):258–263
Cotrufo MF, Gorissen A (1997) Elevated CO2 enhances below-ground C allocation in three perennial grass species at different levels of N availability. New Phytol 137(3):421–431
Cui YX, Fang LC, Deng L, Guo XB, Han F, Ju WL, Wang X, Chen HS, Tan WF, Zhang XC (2019) Patterns of soil microbial nutrient limitations and their roles in the variation of soil organic carbon across a precipitation gradient in an arid and semi-arid region. Sci Total Environ 658:1440–1451
Cui YX, Fang LC, Guo XB, Han F, Ju WL, Ye LP, Wang X, Tan WF, Zhang XC (2019) Natural grassland as the optimal pattern of vegetation restoration in arid and semi-arid regions: Evidence from nutrient limitation of soil microbes. Sci Total Environ 648:388–397
Cui YX, Zhang YL, Duan CJ, Wang X, Zhang XC, Ju WL, Chen HS, Yue SC, Wang YQ, Li SQ, Fang LC (2020) Ecoenzymatic stoichiometry reveals microbial phosphorus limitation decreases the nitrogen cycling potential of soils in semi-arid agricultural ecosystems. Soil Tillage Res 197:10
Davies JAC, Tipping E, Rowe EC, Boyle JF, Pannatier EG, Martinsen V (2016) Long-term P weathering and recent N deposition control contemporary plant-soil C, N, and P. Global Biogeochem Cycles 30(2):231–249
De Kauwe MG, Medlyn BE, Zaehle S, Walker AP, Dietze MC, Wang YP, Luo YQ, Jain AK, El-Masri B, Hickler T, Warlind D, Weng ES, Parton WJ, Thornton PE, Wang SS, Prentice IC, Asao S, Smith B, McCarthy HR, Iversen CM, Hanson PJ, Warren JM, Oren R, Norby RJ (2014) Where does the carbon go? A model-data intercomparison of vegetation carbon allocation and turnover processes at two temperate forest free-air CO2 enrichment sites. New Phytol 203(3):883–899
Deng L, Peng CH, Huang CB, Wang KB, Liu QY, Liu YL, Hai XY, Shangguan ZP (2019) Drivers of soil microbial metabolic limitation changes along a vegetation restoration gradient on the Loess Plateau, China. Geoderma 353:188–200
Dong CC, Wang W, Liu HY, Xu XT, Zeng H (2019) Temperate grassland shifted from nitrogen to phosphorus limitation induced by degradation and nitrogen deposition: Evidence from soil extracellular enzyme stoichiometry. Ecol Ind 101:453–464
Dorodnikov M, Blagodatskaya E, Blagodatsky S, Marhan S, Fangmeier A, Kuzyakov Y (2009) Stimulation of microbial extracellular enzyme activities by elevated CO2 depends on soil aggregate size. Glob Change Biol 15(6):1603–1614
Du EZ, Terrer C, Pellegrini AFA, Ahlstrom A, van Lissa CJ, Zhao X, Xia N, Wu XH, Jackson RB (2020) Global patterns of terrestrial nitrogen and phosphorus limitation. Nat Geosci 13(3):221–226
Ebersberger D, Niklaus PA, Kandeler E (2003) Long term CO2 enrichment stimulates N-mineralisation and enzyme activities in calcareous grassland. Soil Biol Biochem 35(7):965–972
Elser JJ, Bracken MES, Cleland EE, Gruner DS, Harpole WS, Hillebrand H, Ngai JT, Seabloom EW, Shurin JB, Smith JE (2007) Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol Lett 10(12):1135–1142
Fay PA, Prober SM, Harpole WS, Knops JMH, Bakker JD, Borer ET, Lind EM, MacDougall AS, Seabloom EW, Wragg PD, Adler PB, Blumenthal DM, Buckley Y, Chu CJ, Cleland EE, Collins SL, Davies KF, Du GZ, Feng XH, Firn J, Gruner DS, Hagenah N, Hautier Y, Heckman RW, Jin VL, Kirkman KP, Klein J, Ladwig LM, Li Q, McCulley RL, Melbourne BA, Mitchell CE, Moore JL, Morgan JW, Risch AC, Schutz M, Stevens CJ, Wedin DA, Yang LH (2015) Grassland productivity limited by multiple nutrients. Nature Plants 1(7):5
German DP, Weintraub MN, Grandy AS, Lauber CL, Rinkes ZL, Allison SD (2011) Optimization of hydrolytic and oxidative enzyme methods for ecosystem studies. Soil Biol Biochem 43(7):1387–1397
Goll DS, Brovkin V, Parida BR, Reick CH, Kattge J, Reich PB, van Bodegom PM, Niinemets U (2012) Nutrient limitation reduces land carbon uptake in simulations with a model of combined carbon, nitrogen and phosphorus cycling. Biogeosciences 9(9):3547–3569
Guenet B, Lenhart K, Leloup J, Giusti-Miller S, Pouteau V, Mora P, Nunan N, Abbadie L (2012) The impact of long-term CO2 enrichment and moisture levels on soil microbial community structure and enzyme activities. Geoderma 170:331–336
Herold N, Schoning I, Gutknecht J, Alt F, Boch S, Muller J, Oelmann Y, Socher SA, Wilcke W, Wubet T, Schrumpf M (2014) Soil property and management effects on grassland microbial communities across a latitudinal gradient in Germany. Appl Soil Ecol 73:41–50
Hoosbeek MR (2016) Elevated CO2 increased phosphorous loss from decomposing litter and soil organic matter at two FACE experiments with trees. Biogeochemistry 127(1):89–97
Horswill P, O’Sullivan O, Phoenix GK, Lee JA, Leake JR (2008) Base cation depletion, eutrophication and acidification of species-rich grasslands in response to long-term simulated nitrogen deposition. Environ Pollut 155(2):336–349
Hungate BA, Holland EA, Jackson RB, Chapin FS, Mooney HA, Field CB (1997) The fate of carbon in grasslands under carbon dioxide enrichment. Nature 388(6642):576–579
Jackson RB, Banner JL, Jobbagy EG, Pockman WT, Wall DH (2002) Ecosystem carbon loss with woody plant invasion of grasslands. Nature 418(6898):623–626
Jing X, Yang XX, Ren F, Zhou HK, Zhu B, He JS (2016) Neutral effect of nitrogen addition and negative effect of phosphorus addition on topsoil extracellular enzymatic activities in an alpine grassland ecosystem. Appl Soil Ecol 107:205–213
Johnson D, Leake JR, Lee JA, Campbell CD (1998) Changes in soil microbial biomass and microbial activities in response to 7 years simulated pollutant nitrogen deposition on a heathland and two grasslands. Environ Pollut 103(2–3):239–250
Kopacek J, Cosby BJ, Evans CD, Hruska J, Moldan F, Oulehle F, Santruckova H, Tahovska K, Wright RF (2013) Nitrogen, organic carbon and sulphur cycling in terrestrial ecosystems: linking nitrogen saturation to carbon limitation of soil microbial processes. Biogeochemistry 115(1–3):33–51
Loeppmann S, Biagodatskaya E, Pausch J, Kuzyakov Y (2016) Enzyme properties down the soil profile - A matter of substrate quality in rhizosphere and detritusphere. Soil Biol Biochem 103:274–283
Luo L, Meng H, Gu JD (2017) Microbial extracellular enzymes in biogeochemical cycling of ecosystems. J Environ Manage 197:539–549
Luo XZ, Hou EQ, Zang XW, Zhang LL, Yi YF, Wen DZ (2019) Effects of elevated atmospheric CO2 and nitrogen deposition on leaf litter and soil carbon degrading enzyme activities in a Cd-contaminated environment: A mesocosm study. Sci Total Environ 671:157–164
Marklein AR, Houlton BZ (2012) Nitrogen inputs accelerate phosphorus cycling rates across a wide variety of terrestrial ecosystems. New Phytol 193(3):696–704
Meier IC, Pritchard SG, Brzostek ER, McCormack ML, Phillips RP (2015) The rhizosphere and hyphosphere differ in their impacts on carbon and nitrogen cycling in forests exposed to elevated CO2. New Phytol 205(3):1164–1174
Miglietta F, Hoosbeek MR, Foot J, Gigon F, Hassinen A, Heijmans M, Peressotti A, Saarinen T, van Breemen N, Wallen B (2001) Spatial and temporal performance of the MiniFACE (Free Air CO2 Enrichment) system on bog ecosystems in northern and central Europe. Environ Monit Assess 66(2):107–127
Moorhead DL, Rinkes ZL, Sinsabaugh RL, Weintraub MN (2013) Dynamic relationships between microbial biomass, respiration, inorganic nutrients and enzyme activities: informing enzyme-based decomposition models. Frontiers in Microbiology 4:12
Moorhead DL, Sinsabaugh RL, Hill BH, Weintraub MN (2016) Vector analysis of ecoenzyme activities reveal constraints on coupled C, N and P dynamics. Soil Biol Biochem 93:1–7
Mori T (2020) Does ecoenzymatic stoichiometry really determine microbial nutrient limitations? Soil Biol Biochem 146:3
O’Sullivan OS, Horswill P, Phoenix GK, Lee JA, Leake JR (2011) Recovery of soil nitrogen pools in species-rich grasslands after 12 years of simulated pollutant nitrogen deposition: a 6-year experimental analysis. Glob Change Biol 17(8):2615–2628
Peng XQ, Wang W (2016) Stoichiometry of soil extracellular enzyme activity along a climatic transect in temperate grasslands of northern China. Soil Biol Biochem 98:74–84
Philips DA, Fox TC, Six J (2006) Root exudation (net efflux of amino acids) may increase rhizodeposition under elevated CO2. Glob Change Biol 12(3):561–567
Phoenix GK, Booth RE, Leake JR, Read DJ, Grime JP, Lee JA (2003) Effects of enhanced nitrogen deposition and phosphorus limitation on nitrogen budgets of semi-natural grasslands. Glob Change Biol 9(9):1309–1321
Phoenix GK, Booth RE, Leake JR, Read DJ, Grime JP, Lee JA (2004) Simulated pollutant nitrogen deposition increases P demand and enhances root-surface phosphatase activities of three plant functional types in a calcareous grassland. New Phytol 161(1):279–289
Rosinger C, Rousk J, Sanden H (2019) Can enzymatic stoichiometry be used to determine growth-limiting nutrients for microorganisms?–A critical assessment in two subtropical soils. Soil Biol Biochem 128:115–126
Sinsabaugh RL, Shah JJF (2012) Ecoenzymatic Stoichiometry and Ecological Theory. In: Futuyma DJ (ed) Annual Review of Ecology, Evolution, and Systematics, vol 43. Annual Review of Ecology Evolution and Systematics, Annual Reviews, Palo Alto, pp 313–343
Sinsabaugh RL, Turner BL, Talbot JM, Waring BG, Powers JS, Kuske CR, Moorhead DL, Shah JJF (2016) Stoichiometry of microbial carbon use efficiency in soils. Ecol Monogr 86(2):172–189
Souza RC, Solly EF, Dawes MA, Graf F, Hagedorn F, Egli S, Clement CR, Nagy L, Rixen C, Peter M (2017) Responses of soil extracellular enzyme activities to experimental warming and CO2 enrichment at the alpine treeline. Plant Soil 416(1–2):527–537
Thaller MC, Schippa S, Bonci A, Cresti S, Rossolini GM (1997) Identification of the gene (aphA) encoding the class B acid phosphatase/phosphotransferase of Escherichia coli MG1655 and characterization of its product. FEMS Microbiol Lett 146(2):191–198
Turner BL, Wright SJ (2014) The response of microbial biomass and hydrolytic enzymes to a decade of nitrogen, phosphorus, and potassium addition in a lowland tropical rain forest. Biogeochemistry 117(1):115–130
Wang Z, Smyth TJ, Crozier CR, Gehl RJ, Heitman AJ (2018) Yield and Nitrogen Removal of Bioenergy Grasses as Influenced by Nitrogen Rate and Harvest Management in the Coastal Plain Region of North Carolina. Bioenergy Res 11(1):44–53
Waring BG, Weintraub SR, Sinsabaugh RL (2014) Ecoenzymatic stoichiometry of microbial nutrient acquisition in tropical soils. Biogeochemistry 117(1):101–113
Watson R, Albon S, Aspinall R, Austen M, Bardgett B, Bateman I, Berry P, Bird W, Bradbury R, Brown C (2011) UK National Ecosystem Assessment: Technical Report. United Nations Environment Programme World Conservation Monitoring Centre, Cambridge
Yang Y, Liang C, Wang YQ, Cheng H, An SS, Chang SX (2020) Soil extracellular enzyme stoichiometry reflects the shift from P- to N-limitation of microorganisms with grassland restoration. Soil Biol Biochem 149:8
Zaehle S, Jones CD, Houlton B, Lamarque JF, Robertson E (2015) Nitrogen availability reduces CMIP5 projections of twenty-first-century land carbon uptake. J Clim 28(6):2494–2511
Zhang Q, Wang YP, Matear RJ, Pitman AJ, Dai YJ (2014) Nitrogen and phosphorous limitations significantly reduce future allowable CO2 emissions. Geophys Res Lett 41(2):632–637
Acknowledgements
This work was funded by the Natural Environment Research Council, (Grant No. NE/N0100086/1 to IPH, and NE/N010132/1 to GKP) as part of the Phosphorus Limitation And ecosystem responses to Carbon dioxide Enrichment (PLACE) project. We would like to thank Natural England for access to their Wardlow SSSI, Shaun Taylor (Natural England) for help with monolith extraction and transport, and the Peak Park authority for permission to establish the mini-FACE experiment within the Peak District National Park. We are grateful to Dr Gary McClean for work in establishing the experiment, Miranda Vlag for assistance with EEA analysis and Cheryl Hook for soil P analysis.
Funding
This work was funded by the Natural Environment Research Council, (Grant No. NE/N0100086/1 to IPH, and NE/N010132/1 to GKP) as part of the Phosphorus Limitation And ecosystem responses to Carbon dioxide Enrichment (PLACE) project.
Author information
Authors and Affiliations
Contributions
BK—Data curation; Formal analysis; Investigation; Project administration; Software; Visualisation; Writing—original draft; Writing—review & editing. MRH—Conceptualisation; Formal analysis; Funding acquisition; Methodology; Project administration; Resources; Supervision; Roles/Writing—original draft; Writing—review & editing. CRT—Investigation; Methodology; Writing—review & editing. FM—Conceptualisation; Funding acquisition; Methodology; Resources; Writing—review & editing. GKP—Conceptualisation; Data curation; Formal analysis; Funding acquisition; Methodology; Project administration; Resources; Supervision; Validation; Visualisation; Writing—review & editing. IPH—Conceptualisation; Data curation; Formal analysis; Funding acquisition; Methodology; Project administration; Resources; Supervision; Validation; Visualisation; Writing—review & editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Responsible Editor: Jan Mulder.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Keane, J.B., Hoosbeek, M.R., Taylor, C.R. et al. Soil C, N and P cycling enzyme responses to nutrient limitation under elevated CO2. Biogeochemistry 151, 221–235 (2020). https://doi.org/10.1007/s10533-020-00723-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-020-00723-1